推荐产品
化驗
95%
形狀
solid
bp
270 °C (lit.)
mp
62-67 °C (lit.)
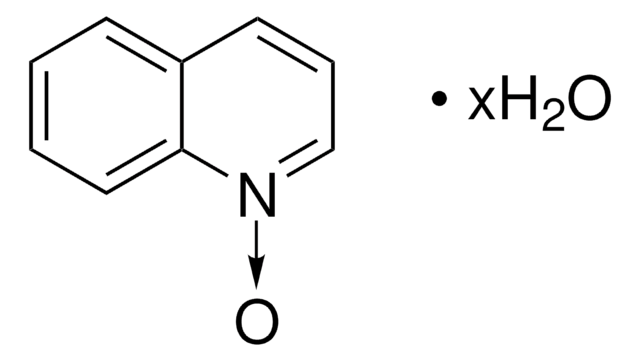
SMILES 字串
[O-][n+]1ccccc1
InChI
1S/C5H5NO/c7-6-4-2-1-3-5-6/h1-5H
InChI 密鑰
ILVXOBCQQYKLDS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
具有[2]轮烷的吡啶N-氧化物轴通过阴离子模板化穿线 - 后续 - 加塞策略合成。
應用
吡啶N-氧化物用于研究吡啶N-氧化物在乙腈中的FTIR光谱。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
289.4 °F - closed cup
閃點(°C)
143 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Xue Gong et al.
Organic letters, 13(7), 1766-1769 (2011-03-11)
A Pd(II)-catalyzed oxidative coupling between pyridine N-oxides and N-substituted indoles via 2-fold C-H bond activation was achieved with high selectivity using Ag(2)CO(3) as an oxidant.
Masahito Murai et al.
Chemical communications (Cambridge, England), 48(61), 7622-7624 (2012-06-26)
Gold(I)-catalysed tandem oxygen-transfer/cycloisomerisation reaction of 2-(2-propynyl)pyridine N-oxides provides an atom-economical route to indolizinone frameworks.
Munawar Hussain et al.
Organic letters, 15(1), 54-57 (2012-12-22)
The synthesis of optically active piperidines by enantioselective addition of aryl Grignard reagents to pyridine N-oxides and lithium binolate followed by reduction is reported for the first time. The reaction results in high yields (51-94%) in combination with good ee
Jinshui Chen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(30), 7268-7276 (2009-07-07)
Optically active chiral alkyl chlorides are valuable compounds because of their bioactivity and versatile synthetic utility. Accordingly, the ring opening of epoxides with a chloride nucleophile stands as an important goal in asymmetric catalysis. We describe herein recent advances in
Highly efficient gold nanoparticle catalyzed deoxygenation of amides, sulfoxides, and pyridine N-oxides.
Yusuke Mikami et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(6), 1768-1772 (2011-01-29)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门