推荐产品
方案
≥99.0%
表单
powder
mp
184-186 °C
储存温度
2-8°C
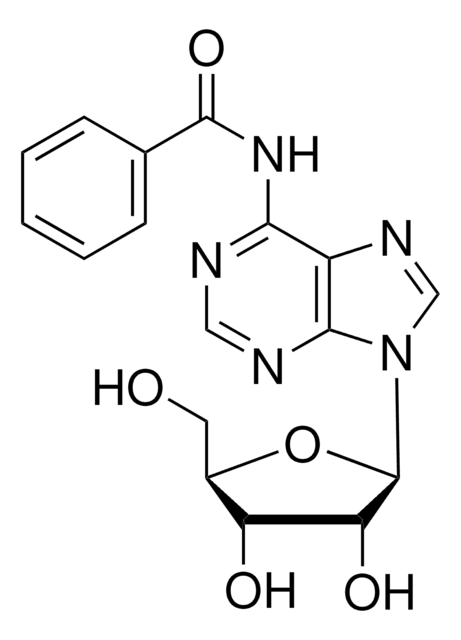
SMILES字符串
OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(NCc4ccccc4)ncnc23
InChI
1S/C17H19N5O4/c23-7-11-13(24)14(25)17(26-11)22-9-21-12-15(19-8-20-16(12)22)18-6-10-4-2-1-3-5-10/h1-5,8-9,11,13-14,17,23-25H,6-7H2,(H,18,19,20)/t11-,13-,14-,17-/m1/s1
InChI key
MRPKNNSABYPGBF-LSCFUAHRSA-N
基因信息
human ... ADORA3(140)
rat ... Adora1(29290) , Adora2a(25369) , Adora3(25370)
正在寻找类似产品? 访问 产品对比指南
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
R G Luiten et al.
Nucleic acids research, 11(22), 8073-8085 (1983-11-25)
From in vitro protein synthesis studies and nucleotide sequence analysis it has been deduced that, unlike the major coat proteins of the hitherto studied filamentous bacterial viruses Ff (M13, fd and f1), IKe and Pf1, the major coat protein of
P Mlejnek
Journal of cellular biochemistry, 83(4), 678-689 (2001-12-18)
As an extension of our recently published work (Mlejnek and Kuglík [2000] J. Cell. Biochem. 77:6-17), the role of caspases in N(6)-benzylaminopurine riboside (BAPR)-induced apotosis in HL-60 cells was evaluated in this study. Here, BAPR-induced apoptosis was accompanied by activation
Karel Dolezal et al.
Bioorganic & medicinal chemistry, 15(11), 3737-3747 (2007-04-10)
Cytokinin activity of forty-eight 6-benzyladenosine derivatives at both the receptor and cellular levels as well as their anticancer properties were compared in various in vitro assays. The compounds were prepared by the condensation of 6-chloropurine riboside with corresponding substituted benzylamines
B C Froehler et al.
Nucleic acids research, 11(22), 8031-8036 (1983-11-25)
Sterically hindered N6-dialkylformamidine protected deoxyadenosine is more stable to acidic depurination than N6-benzoyldeoxyadenosine and is potentially a valuable protecting group in the synthesis of deoxyoligonucleotides.
Petr Dolezel et al.
Toxicology in vitro : an international journal published in association with BIBRA, 24(8), 2079-2083 (2010-07-20)
Cytotoxicity of two halogen derivatives of N⁶-benzyladenosine (BAPR), N⁶-(3-iodobenzyl)-adenosine (I-BAPR) and 2-chloro-N⁶-(3-iodobenzyl)-adenosine (Cl-I-BAPR), was tested in human leukemia U937 cell line. Our results revealed that their cytotoxicity was surprisingly low. I-BAPR and also Cl-I-BAPR induced cell death with morphological and
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门