所有图片(1)
About This Item
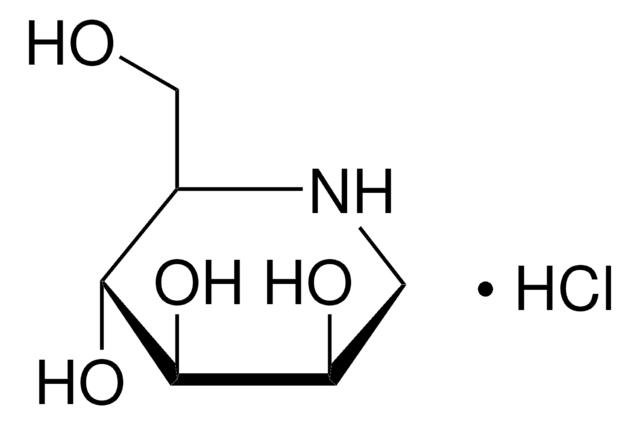
经验公式(希尔记法):
C8H15NO3
CAS号:
分子量:
173.21
Beilstein:
4175740
MDL號碼:
分類程式碼代碼:
51102829
PubChem物質ID:
NACRES:
NA.85
推荐产品
生物源
Metarrhizium anisopliae
品質等級
化驗
≥98% (TLC)
形狀
lyophilized powder
儲存條件
(Keep container tightly closed in a dry and well-ventilated place.)
顏色
white to faint yellow
溶解度
H2O: soluble 1 mg/mL
抗生素活性譜
neoplastics
作用方式
enzyme | inhibits
儲存溫度
2-8°C
SMILES 字串
O[C@@H]1CCCN2C[C@@H](O)[C@@H](O)C12
InChI
1S/C8H15NO3/c10-5-2-1-3-9-4-6(11)8(12)7(5)9/h5-8,10-12H,1-4H2/t5-,6-,7?,8-/m1/s1
InChI 密鑰
FXUAIOOAOAVCGD-DCDLSZRSSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Swainsonine is an indolizidine alkaloid from the plant Metarrhizium anisopliae that is used as a potent α-mannosidase inhibitor. Product S8195 has been used in chemical inhibition assays of CHO Lec2 cells to inhibit glycosylation .
苦马豆素是各种α-甘露糖苷酶的有效抑制剂,特别是α-甘露糖苷酶II。它能够抑制糖蛋白加工,也可作为免疫调节剂。
生化/生理作用
Swainsonine is a potent α-mannosidase inhibitor. It also has antimetastatic, antiproliferative, and immunomodulatory activity . It also inhibits glycoprotein processing.
包裝
1MG
準備報告
Soluble in water, methanol, DMSO
其他說明
Keep container tightly closed in a dry and well-ventilated place.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Shen Shen et al.
The Journal of biological chemistry, 286(15), 13532-13540 (2011-02-19)
Sialylated glycans serve as cell surface attachment factors for a broad range of pathogens. We report an atypical example, where desialylation increases cell surface binding and infectivity of adeno-associated virus (AAV) serotype 9, a human parvovirus isolate. Enzymatic removal of
Guo-Dong Yang et al.
Toxicon : official journal of the International Society on Toxinology, 60(1), 44-49 (2012-04-10)
Endophytic Undifilum oxytropis found within toxic locoweeds (Astragalus and Oxytropis spp.) produces the indolizidine alkaloid swainsonine, which is responsible for locoism in grazing animals. The aim of the current study is to establish an easy and accurate method for the
Julien Louvel et al.
Organic letters, 13(24), 6452-6455 (2011-11-16)
The asymmetric synthesis of (-)-swainsonine and (-)-8-epi-swainsonine is reported through the addition of either the allenylzinc or the allenyl lithio cyanocuprate reagents derived from [3-(methoxymethoxy)prop-1-ynyl]trimethylsilane to enantiopure α,β-dialkoxy N-tert-butanesulfinylimines derived from d-erythronolactone.
Fábio S Mendonça et al.
Journal of veterinary diagnostic investigation : official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc, 24(1), 90-95 (2012-03-01)
A disease of the nervous system is reported in goats in the semiarid region of northeastern Brazil. Histological examination showed diffuse vacuolation of neurons and epithelial cells of the pancreas, thyroid, renal tubules, and liver. The swainsonine-containing plant Ipomoea verbascoidea
Zhaocai Li et al.
International journal of biological sciences, 8(3), 394-405 (2012-03-07)
Swainsonine (1, 2, 8-trihyroxyindolizidine, SW), a natural alkaloid, has been reported to exhibit anti-cancer activity on several mouse models of human cancer and human cancers in vivo. However, the mechanisms of SW-mediated tumor regression are not clear. In this study
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门