推荐产品
等级
analytical standard
质量水平
表单
powder or crystals
技术
HPLC: suitable
gas chromatography (GC): suitable
应用
forensics and toxicology
pharmaceutical (small molecule)
veterinary
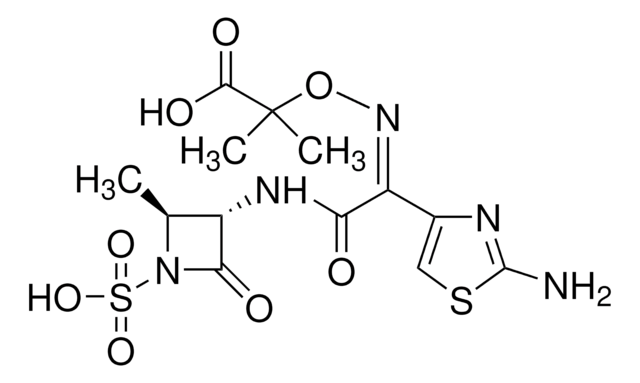
SMILES字符串
C[C@H]1[C@H](NC(=O)\C(=N/OC(C)(C)C(O)=O)c2csc(N)n2)C(=O)N1S(O)(=O)=O
InChI
1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1
InChI key
WZPBZJONDBGPKJ-VEHQQRBSSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
Aztreonam belongs to the group of monocyclic β-lactam antibiotics, isolated from Chromobacterium violaceum. It is used against gram-negative bacterial infections, such as infections of the bladder, kidneys, bone, and urinary tract. It acts by binding to the penicillin-binding protein-3 (PBP3) and inhibiting the synthesis of bacterial cell walls causing cell lysis and death.
应用
The analytical standard can be used as follows:
- Determination of aztreonam in two simulated lung fluid solutions― artificial lysosomal fluid and gamble solution by a UV spectroscopic method
- Multi-residue analysis of eight β-lactams by ultra-high performance liquid chromatography (UHPLC) combined with a photo-diode array (PDA) detector in human plasma & serum samples
- Simultaneous quantitative analysis of nine β-lactams by UHPLC along with tandem mass spectrometry (MS/MS) in human plasma samples
其他说明
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
储存分类代码
11 - Combustible Solids
WGK
WGK 2
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Neonatal and Pediatric Pharmacology: Therapeutic Principles in Practice (2010)
The Antimicrobial Drugs (2000)
Michael N Dudley et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 56(9), 1301-1309 (2013-01-22)
Widespread resistance in Enterobacteriaceae and Pseudomonas aeruginosa to cephalosporin and monobactam antibiotics due to extended-spectrum β-lactamases (ESBLs) has resulted in the need for reassessment of the interpretative criteria (breakpoints) established for these agents more than 2 decades ago. Following extensive
H Mattie
Clinical pharmacokinetics, 26(2), 99-106 (1994-02-01)
Plasma concentrations of aztreonam follow a 2-compartment open model with a distribution half-life of 0.20 hours after intravenous injection. The volume of distribution at steady-state (Vss) after intravenous and intramuscular injection is about 0.16 L/kg (0.42 L/kg for free drug).
Kristen Zeitler et al.
American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists, 69(2), 107-115 (2012-01-05)
The pharmacology, safety, efficacy, pharmacokinetics, pharmacodynamics, current place in therapy, and potential future therapeutic uses of inhaled aztreonam are reviewed. Inhaled aztreonam, a newly formulated lysine salt of the original monobactam antibiotic, is approved for the treatment of respiratory symptoms
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门