推荐产品
等级
pharmaceutical primary standard
API类
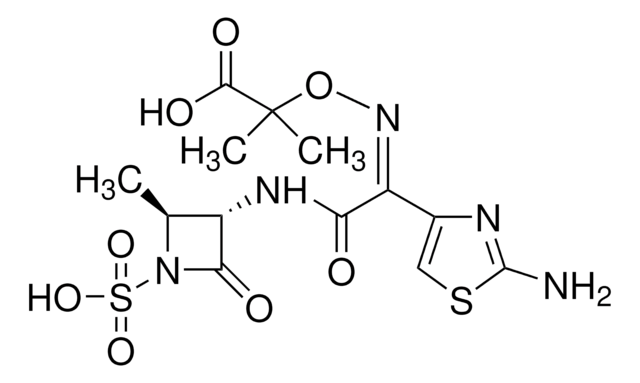
aztreonam
制造商/商品名称
USP
应用
pharmaceutical (small molecule)
包装形式
neat
储存温度
−20°C
SMILES字符串
C[C@H]1[C@H](NC(=O)\C(=N/OC(C)(C)C(O)=O)c2csc(N)n2)C(=O)N1S(O)(=O)=O
InChI
1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1
InChI key
WZPBZJONDBGPKJ-VEHQQRBSSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
应用
Aztreonam USP reference standard is meant to use for specified quality tests and assays.
Also used to prepare standard and system suitability solutions for assay according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard and system suitability solutions for assay according to the given below monographs of United States Pharmacopeia (USP):
- Aztreonam
- Aztreonam for Injection
- Aztreonam Injection
分析说明
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他说明
Sales restrictions may apply.
储存分类代码
11 - Combustible Solids
WGK
WGK 2
闪点(°F)
Not applicable
闪点(°C)
Not applicable
Aztreonam for Injection
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 39(3), 463-463 (2018)
Aztreonam Injection
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 39(3), 462-462 (2018)
Michael N Dudley et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 56(9), 1301-1309 (2013-01-22)
Widespread resistance in Enterobacteriaceae and Pseudomonas aeruginosa to cephalosporin and monobactam antibiotics due to extended-spectrum β-lactamases (ESBLs) has resulted in the need for reassessment of the interpretative criteria (breakpoints) established for these agents more than 2 decades ago. Following extensive
H Mattie
Clinical pharmacokinetics, 26(2), 99-106 (1994-02-01)
Plasma concentrations of aztreonam follow a 2-compartment open model with a distribution half-life of 0.20 hours after intravenous injection. The volume of distribution at steady-state (Vss) after intravenous and intramuscular injection is about 0.16 L/kg (0.42 L/kg for free drug).
Kristen Zeitler et al.
American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists, 69(2), 107-115 (2012-01-05)
The pharmacology, safety, efficacy, pharmacokinetics, pharmacodynamics, current place in therapy, and potential future therapeutic uses of inhaled aztreonam are reviewed. Inhaled aztreonam, a newly formulated lysine salt of the original monobactam antibiotic, is approved for the treatment of respiratory symptoms
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门