推荐产品
品質等級
產品線
Novabiochem®
化驗
≥98% (TLC)
≥98.0% (acidimetric)
≥99.0% (HPLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
mp
164-175 °C
應用
peptide synthesis
官能基
thiol
儲存溫度
−20°C (−15°C to −25°C)
InChI
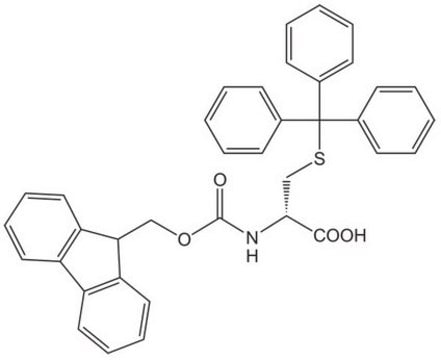
1S/C37H31NO4S/c39-35(40)34(38-36(41)42-24-33-31-22-12-10-20-29(31)30-21-11-13-23-32(30)33)25-43-37(26-14-4-1-5-15-26,27-16-6-2-7-17-27)28-18-8-3-9-19-28/h1-23,33-34H,24-25H2,(H,38,41)(H,39,40)/p-1/t34-/m0/s1
InChI 密鑰
KLBPUVPNPAJWHZ-UMSFTDKQSA-M
一般說明
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Fmoc SPPS of Cysteine-Containing Peptides
Literature references
[1] S. N. McCurdy (1989) Pept. Res., 2, 147.
[2] T. Kaiser, et al. (1996) Tetrahedron Lett., 37, 1187.
[3] Y. X. Han, et al. (1997) J. Org. Chem., 62, 4307.
[4] Y. N. Angell (2002) J. Peptide Res., 5, 292.
應用
- On-resin synthesis of cyclic peptides via tandem N-to-S acyl migration and intramolecular thiol additive-free native chemical ligation: Discusses the use of Fmoc-Cys(Trt)-OH in the synthesis of cyclic peptides, highlighting the efficiency of the resin synthesis method. (Serra et al., 2020).
- Selective Bi‐directional Amide Bond Cleavage of N‐Methylcysteinyl Peptide: The study utilized Fmoc-Cys(Trt)-OH in exploring selective bi-directional amide bond cleavage in peptides, providing insights into controlled peptide modification. (Qiu et al., 2014).
聯結
分析報告
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.5 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Cys (Trt) -OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Cys(Trt)-Cys(Trt)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Cys-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (HPLC): ≤ 0.2 %
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 98.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法律資訊
未找到合适的产品?
试试我们的产品选型工具.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
实验方案
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
相关内容
Purer Fmocs Means Purer Peptides
Purer Fmocs Means Purer Peptides
Purer Fmocs Means Purer Peptides
Purer Fmocs Means Purer Peptides
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门