所有图片(1)
About This Item
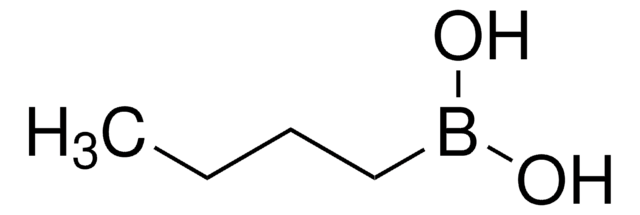
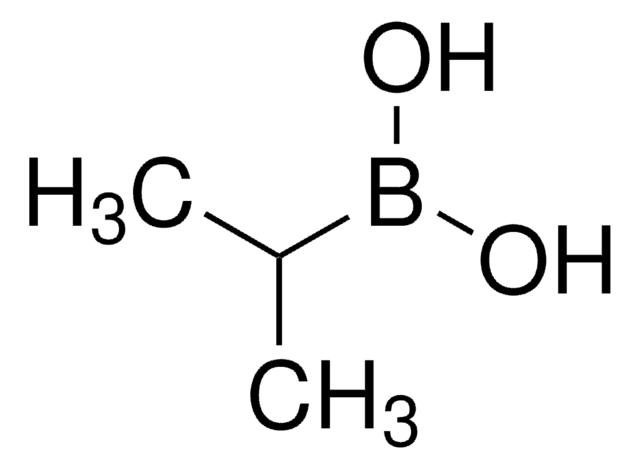
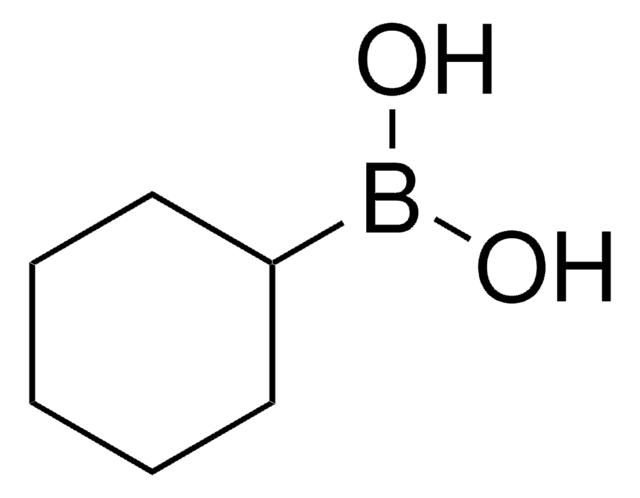
线性分子式:
(CH3)2CHCH2B(OH)2
CAS号:
分子量:
101.94
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥95.0%
形狀
solid
mp
108-111 °C (lit.)
SMILES 字串
CC(C)CB(O)O
InChI
1S/C4H11BO2/c1-4(2)3-5(6)7/h4,6-7H,3H2,1-2H3
InChI 密鑰
ZAZPDOYUCVFPOI-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(2-甲基丙基)硼酸可用作:
它还可用作以下反应的反应物:
- 通过与4-溴异喹啉的Suzuki-Miyaura偶联反应制备4-异丁基异喹啉的反应物。
- 与氢氧化铝和硼酸一起用作苯乙烯聚合的催化剂。
它还可用作以下反应的反应物:
- 铜催化的交叉偶联反应。
- 通过铱催化的C-H硼化反应合成聚硼基烷。
- 杂取代二氮杂硼杂环戊二烯和硼杂环化合物的制备。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Study of Rate-accelerating of Aluminum Hydroxide, Boric Acid, and (2-Methylpropyl) Boronic Acid for Atom Transfer Radical Polymerization of Styrene
Luo Yu-tai, et al.
Journal of Xiamen University (Natural Science), 47(1), 63-63 (2008)
Steven A Rossi et al.
Organic letters, 15(9), 2314-2317 (2013-04-25)
For the first time, a general catalytic procedure for the cross-coupling of primary amides and alkylboronic acids is demonstrated. The key to the success of this reaction was the identification of a mild base (NaOSiMe3) and oxidant (di-tert-butyl peroxide) to
Takeshi Yamamoto et al.
Organic letters, 21(16), 6235-6240 (2019-08-07)
Pyrazolylaniline serves as a temporary directing group attached to the boron atom of alkylboronic acids in Ir-catalyzed C(sp3)-H borylation. The reaction takes place at α-, β-, and γ-C-H bonds, giving polyborylated products including di-, tri-, tetra-, and even pentaborylalkanes. α-C-H
Solution-state 15N NMR and solid-state single-crystal XRD study of heterosubstituted diazaboroles and borinines prepared via an effective and simple microwave-assisted solvent-free synthesis
Slabber CA, et al.
Journal of Organometallic Chemistry, 723, 122-128 (2013)
Benjamin M Reeves et al.
Angewandte Chemie (International ed. in English), 58(44), 15697-15701 (2019-09-06)
A transition-metal-free reductive hydroxymethylation reaction has been developed, enabling the preparation of tetrahydroisoquinolines bearing C4-quaternary centers from the corresponding isoquinolines. Deuterium labelling studies and control experiments enable a potential mechanism to be elucidated which features a key Cannizzaro-type reduction followed
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门