推荐产品
品質等級
化驗
99%
形狀
liquid
包含
copper as stabilizer
折射率
n20/D 1.661 (lit.)
bp
120-121 °C/15 mmHg (lit.)
mp
9-10 °C (lit.)
密度
2.203 g/mL at 25 °C (lit.)
SMILES 字串
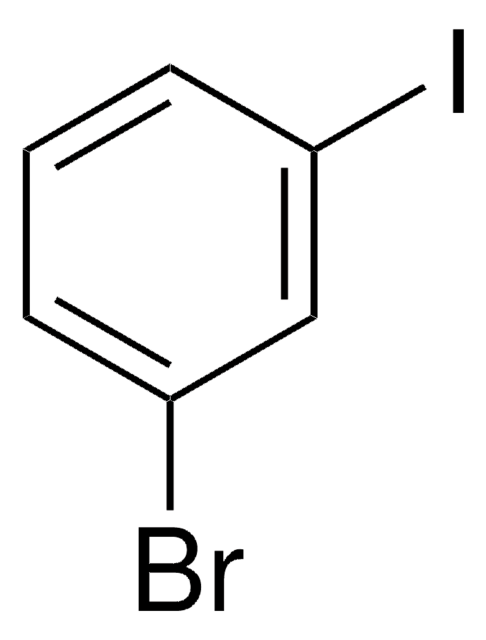
Brc1ccccc1I
InChI
1S/C6H4BrI/c7-5-3-1-2-4-6(5)8/h1-4H
InChI 密鑰
OIRHKGBNGGSCGS-UHFFFAOYSA-N
一般說明
2-溴碘苯是一种亲电试剂,可与氨基官能化有机锌试剂进行钯催化交叉偶联反应。
應用
2-溴代二苯可用于合成二芳基胺。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Routes toward enantiopure 2-substituted indolines: an overview
Anas, et al.
Tetrahedron Asymmetry, 20, 2193-2199 (2009)
Synthesis of 2-substituted indolines using sequential Pd-catalyzed processes
Deboves, et al.
Journal of the Chemical Society. Perkin Transactions 1, 733-736 (2002)
María E Budén et al.
The Journal of organic chemistry, 74(12), 4490-4498 (2009-05-23)
The synthesis of a series of substituted 9H-carbazoles by the photostimulated S(RN)1 substitution reaction with diarylamines as starting substrate was performed. The diarylamines were obtained by two approaches, the Pd-catalyzed reactions (Buchwald-Hartwig) or Cu-catalyzed reactions of 2-haloanilines with aryl halides
Lei Liu et al.
Journal of pharmaceutical and biomedical analysis, 117, 325-332 (2015-09-29)
The current study reports the development and validation of a stability-indicating reversed phase HPLC method for the separation and identification of potential impurities in vortioxetine, a recently developed antidepressant. The structures of a new compound and four process-related impurities formed
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门