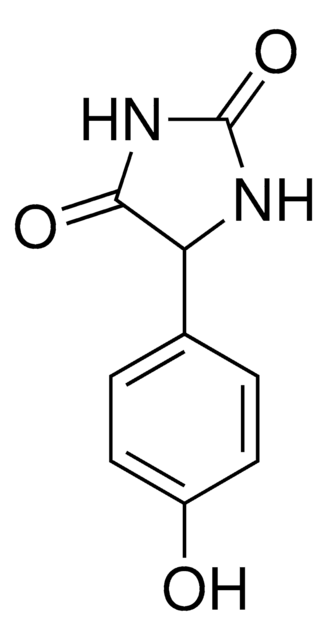
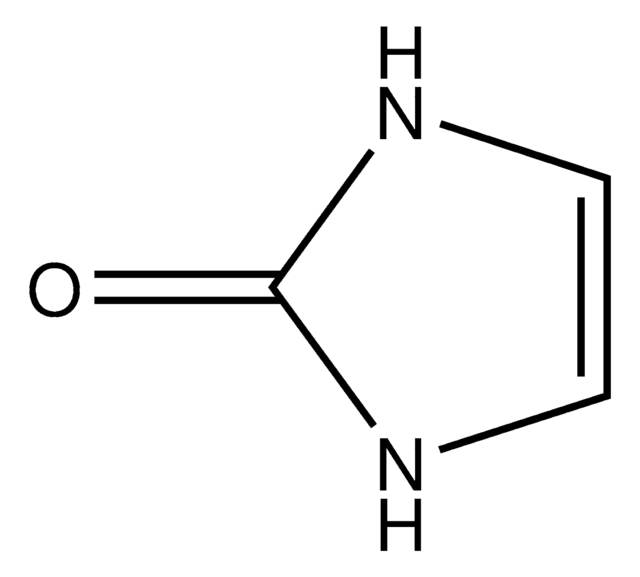
价格与库存信息目前不能提供
推荐产品
质量水平
方案
98%
表单
powder
mp
218-220 °C (lit.)
SMILES字符串
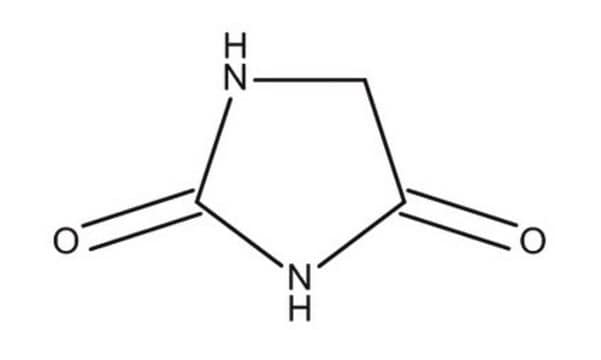
O=C1CNC(=O)N1
InChI
1S/C3H4N2O2/c6-2-1-4-3(7)5-2/h1H2,(H2,4,5,6,7)
InChI key
WJRBRSLFGCUECM-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
其他客户在看
June Izquierdo et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(53), 12431-12438 (2019-07-19)
A bifunctional amine/squaramide catalyst promoted direct aldol addition of an hydantoin surrogate to pyridine 2-carbaldehyde N-oxides to afford adducts bearing two vicinal tertiary/quaternary carbons in high diastereo- and enantioselectivity (d.r. up to >20:1; ee up to 98 %) is reported. Acid
Effect of treatment with compressed CO2 and propane on D-hydantoinase activity
Andrade JM, et al.
Journal of Supercritical Fluids, 46(2), 342-350 (2008)
Y Thirupathi Reddy et al.
Bioorganic & medicinal chemistry letters, 20(2), 600-602 (2009-12-17)
A series of (Z)-5-((N-benzyl-1H-indol-3-yl)methylene)imidazolidine-2,4-dione (9a-9m) and 5-((N-benzyl-1H-indol-3-yl)methylene)pyrimidine-2,4,6(1H,3H,5H)-trione (10a-10i) derivatives that incorporate a variety of aromatic substituents in both the indole and N-benzyl moieties have been synthesized. These analogs were evaluated for their radiosensitization activity against the HT-29 cell line. Three
Suguru Nishinami et al.
International journal of biological macromolecules, 114, 497-503 (2018-03-06)
Allantoin is widely used in pharmaceutical and cosmetic products, and is composed of a hydantoin ring and a ureido group. Recent reports showed that allantoin suppresses thermal aggregation of hen egg white lysozyme (LYZ). However, structural insight into the properties
Mohammad A Khanfar et al.
Journal of medicinal chemistry, 53(24), 8534-8545 (2010-11-19)
Dysregulation of glycogen synthase kinase (GSK-3β) is implicated in the pathophysiology of many diseases, including type-2 diabetes, stroke, Alzheimer's, and others. A multistage virtual screening strategy designed so as to overcome known caveats arising from the considerable flexibility of GSK-3β
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门