392979
15-Hydroxypentadecanoic acid
97%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
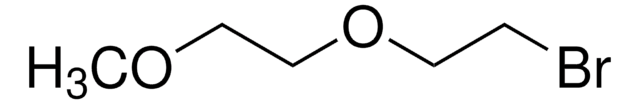
Formule linéaire :
HO(CH2)14CO2H
Numéro CAS:
Poids moléculaire :
258.40
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
97%
Pf
85-89 °C (lit.)
Groupe fonctionnel
carboxylic acid
hydroxyl
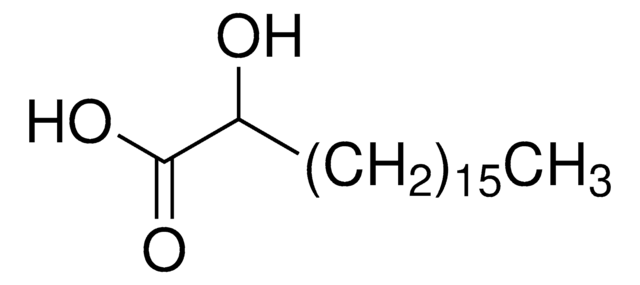
Chaîne SMILES
OCCCCCCCCCCCCCCC(O)=O
InChI
1S/C15H30O3/c16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15(17)18/h16H,1-14H2,(H,17,18)
Clé InChI
BZUNJUAMQZRJIP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
15-Hydroxypentadecanoic acid is an ω-hydroxy acid. One of the method reported for its synthesis is from 1,12-dodecanolide. It is reported to be one of the bioactive component in Tagetes erecta L. leaf and flower extract.
15-Hydroxypentadecanoic acid undergoes lactonization reaction catalyzed by Mucor javanicus L46 and Mucor miehei to afford macrocyclic mono- and oligolactone derivatives. Its lipase-catalyzed synthesis from 15-tetracosenoic acid in Malania Olcifera Chum oil has been proposed. It also participates in the biosynthesis of pentadecanolide.
Application
15-Hydroxypentadecanoic acid is suitable reagent used in the following studies:
- As an internal standard in the quantification of formation of 11-hydroxylauric acid by gas chromatography.
- In the synthesis of [16-14C]16DCA (DCA= dicarboxylic acid) by one-carbon elongation procedure at C15.
- As an internal standard for the normalization of intensities in the mass spectra of plant cutin polymer.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Sacha Ferdinandusse et al.
Journal of lipid research, 45(6), 1104-1111 (2004-04-03)
Dicarboxylic acids (DCAs) are omega-oxidation products of monocarboxylic acids. After activation by a dicarboxylyl-CoA synthetase, the dicarboxylyl-CoA esters are shortened via beta-oxidation. Although it has been studied extensively where this beta-oxidation process takes place, the intracellular site of DCA oxidation
Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones.
Antczak U, et al.
Enzyme and Microbial Technology, 13(7), 589-593 (1991)
Lipase catalyzed synthesis of pentadecanolide from 15-hydroxypentadecanoic acid.
Pan XB, et al.
Chinese Journal of Applied Chemistry / Ying Yong Hua Xue, 21(8), 850-852 (2004)
Zeolite-catalyzed macrolactonization of Ookoshi T and Onaka M. ω-hydroxyalkanoic acids in a highly concentrated solution.
Ookoshi T and Onaka M.
Tetrahedron Letters, 39(3), 293-296 (1998)
Jamal Mustafa et al.
Lipids, 39(2), 167-172 (2004-05-12)
Derivatives of podophyllotoxin were prepared by coupling 10 FA with the C4-alpha-hydroxy function of podophyllotoxin. The coupling reactions between FA and podophyllotoxin were carried out by dicyclohexylcarbodiimide in the presence of a catalytic amount of dimethylaminopyridine to produce quantitative yields
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![2-[2-(2-Methoxyethoxy)ethoxy]acetic acid technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)