Wichtige Dokumente
930962
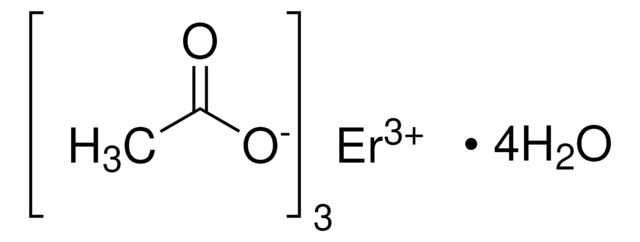
Yttrium(III) acetate tetrahydrate
99.99% trace rare earth metals basis
Synonym(e):
Acetic acid yttrium(3+) salt, Yttrium triacetate
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
99.99% trace rare earth metals basis
Form
powder
Verunreinigungen
≤150 ppm trace rare earth metals
≤500 ppm trace metals
mp (Schmelzpunkt)
350 °C (Decomp.)
Löslichkeit
H2O: soluble (lit.)
Dichte
1.5 g/cm3
Allgemeine Beschreibung
Anwendung
A major application of high-purity yttrium acetate is in the synthesis of sodium yttrium fluoride (NaYF4) nanoparticles. Typically, in these syntheses, yttrium acetate is mixed with oleic acid in octadecene and heated to form Y(oleate)3, which is reacted with ammonium fluoride and sodium hydroxide in methanol at modest temperatures (e.g. 50 C) to form NaYF4 nanoparticles. This synthesis offers great control over particle size and crystallinity and allows for easy incorporation rare-earth metal dopants.
Lanthanide-doped NaYF4 nanoparticles are one of the most studied materials for up conversion. These nanoparticles, which can convert two photons of near-infrared (NIR) light into visible light, have important in-vivo applications because of the deep tissue penetration abilities of NIR. For example, these nanoparticles have been used for in-vivo Zn2+ optical sensing, in-vivo ratiometric sensing of lymphatic inflammation,, and in-vivo sensing of peroxynitrite.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.