90827
Tributylphosphin
≥93.5% (Tri-N-butylphosphine, GC)
Synonym(e):
TBP
About This Item
Empfohlene Produkte
Dampfdichte
9 (vs air)
Qualitätsniveau
Assay
≥93.5% (Tri-N-butylphosphine, GC)
≥97% (Tri-N-butylphospine + isomers)
Form
liquid
Selbstzündungstemp.
392 °F
Eignung der Reaktion
reaction type: Acetylations
reagent type: ligand
Brechungsindex
n20/D 1.462 (lit.)
n20/D 1.463
bp
150 °C/50 mmHg (lit.)
Dichte
0.81 g/mL at 25 °C (lit.)
Funktionelle Gruppe
phosphine
SMILES String
CCCCP(CCCC)CCCC
InChI
1S/C12H27P/c1-4-7-10-13(11-8-5-2)12-9-6-3/h4-12H2,1-3H3
InChIKey
TUQOTMZNTHZOKS-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
It may be used in the following processes:
- As reducing agent for alkyl disulfides and aromatic disulfides.
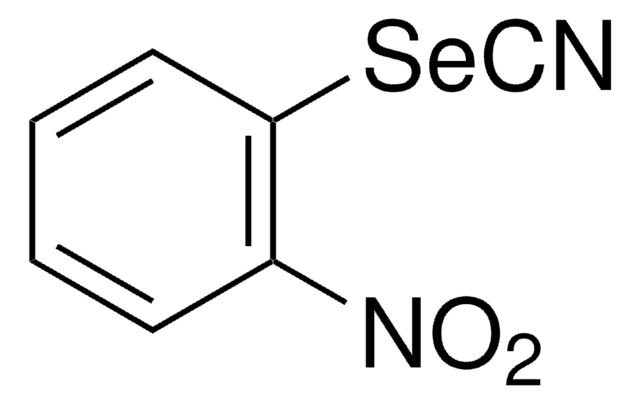
- As catalyst for the synthesis of 2-substituted 1,3-benzoselenazoles.
- As promoter for the ring opening of epoxides and aziridines with nucleophiles.
- As a reagent in the preparation of 6-substituted penicillanate esters by reduction of 6-bromo-6-substituted penicillanate esters in high diastereoselectivity.
- As a catalyst in the acylation reaction of alcohols.
- As a catalyst to prepare rotaxanes by the acylation of corresponding pseudorotaxanes using 3,5-dimethylbenzoic anhydride.
- As a catalyst to prepare vinyl thioethers by the Michael addition of ethanethiol to various alkynyl ketones.
- As a promoter in the conjugate addition of non-nucleophilic N-containing compounds with Michael acceptors.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1A
Lagerklassenschlüssel
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 2
Flammpunkt (°F)
242.6 °F - closed cup
Flammpunkt (°C)
117 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.