추천 제품
Grade
analytical standard
Quality Level
분석
≥98.5% (HPLC)
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
불순물
≤12.0% water
응용 분야
forensics and toxicology
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
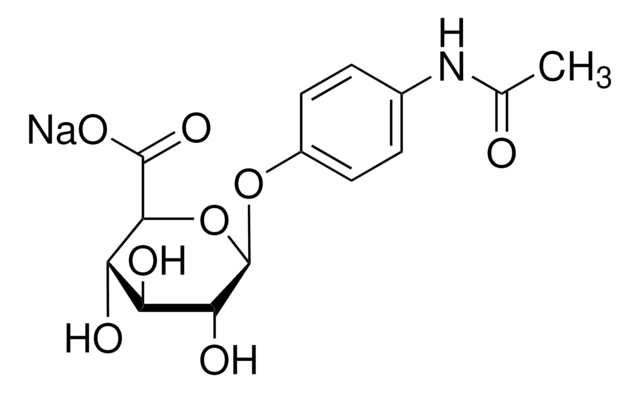
SMILES string
O[C@@H]1[C@@H](O)[C@H](OC2=CC=C(NC(C)=O)C=C2)O[C@H](C(O)=O)[C@H]1O
InChI
1S/C14H17NO8/c1-6(16)15-7-2-4-8(5-3-7)22-14-11(19)9(17)10(18)12(23-14)13(20)21/h2-5,9-12,14,17-19H,1H3,(H,15,16)(H,20,21)/t9-,10-,11+,12-,14+/m0/s1
InChI key
IPROLSVTVHAQLE-BYNIDDHOSA-N
관련 카테고리
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Choose from one of the most recent versions:
이미 열람한 고객
Nicole J Barshop et al.
Journal of pediatric gastroenterology and nutrition, 52(2), 198-202 (2011-01-18)
: The aim of the study was to evaluate UDP-glucuronyltransferase activity and the pharmacokinetics of a single oral dose of acetaminophen (APAP) in children with nonalcoholic fatty liver disease (NAFLD). : Twelve boys 10 to 17 years old with biopsy-proven
Sifeng Mao et al.
Lab on a chip, 12(1), 219-226 (2011-11-19)
In this work, we developed a microfluidic device for the imitation of drug metabolism in human liver and its cytotoxicity on cells. The integrated microfluidic device consists of three sections: (1) bioreactors containing poly(ethylene) glycol (PEG) hydrogel encapsulated human liver
Carolina I Ghanem et al.
Biochemical pharmacology, 77(10), 1621-1628 (2009-05-12)
Development of resistance to toxic effects of acetaminophen (APAP) was reported in rodents and humans, though the mechanism is only partially understood. We examined in rats the effect of administration with subtoxic daily doses (0.2, 0.3, and 0.6g/kg, i.p.) of
Karel Allegaert et al.
Therapeutic drug monitoring, 31(4), 411-415 (2009-06-06)
Compared with phase I isoenzymes, data on isoenzyme-specific phenotypic activity of uridine diphosphate glucuronosyltransferase (UGT) and its covariates in neonates are limited. In vivo observations on morphine, paracetamol (acetaminophen), and propofol disposition throughout childhood confirm the overall low-glucuronidation activity in
Jinchun Sun et al.
Drug metabolism letters, 3(3), 130-136 (2009-08-26)
A LC/MS-based metabolomic assay was utilized to investigate a drug's excretion kinetic profile in urine so that the drug toxicity information could be obtained. Groups of 10 male Sprague-Dawley rats per dose were orally gavaged with a single dose of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.