G6104
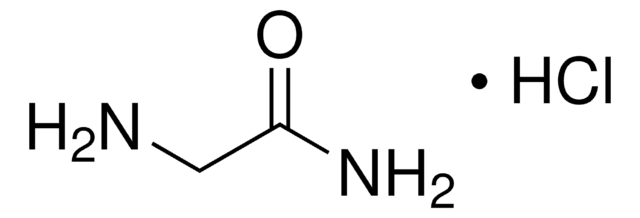
Glycinamide hydrochloride
98%
동의어(들):
2-Aminoacetamide hydrochloride, Aminoacetamide hydrochloride, Glycine amide hydrochloride
로그인조직 및 계약 가격 보기
모든 사진(3)
크기 선택
보기 변경
5 G
₩85,159
25 G
₩90,538
About This Item
Linear Formula:
NH2CH2CONH2 · HCl
CAS Number:
Molecular Weight:
110.54
Beilstein:
3554199
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
mp
204 °C (dec.) (lit.)
SMILES string
Cl.NCC(N)=O
InChI
1S/C2H6N2O.ClH/c3-1-2(4)5;/h1,3H2,(H2,4,5);1H
InChI key
WKNMKGVLOWGGOU-UHFFFAOYSA-N
애플리케이션
Buffer useful in the physiological pH range.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Samir Abdurahman et al.
Antimicrobial agents and chemotherapy, 52(10), 3737-3744 (2008-07-23)
Upon maturation of the human immunodeficiency virus type 1 (HIV-1) virion, proteolytic cleavage of the Gag precursor protein by the viral protease is followed by morphological changes of the capsid protein p24, which will ultimately transform the virus core from
Irene M Lagoja et al.
Chemistry & biodiversity, 2(7), 923-927 (2006-12-29)
A possible reaction mechanism for the dehydration of glycinamide (3) and N,N'-diformylurea (4) yielding hypoxanthine (2) has been investigated. Furthermore, a potential prebiotic route converting hypoxanthine (2) into adenine (1) via phosphate activation followed by substitution reaction with NH3 was
Stefan Glatzel et al.
Chemical communications (Cambridge, England), 46(25), 4517-4519 (2010-05-21)
Homopolymers of N-acryloyl glycinamide were prepared by reversible addition-fragmentation chain transfer polymerization in water. The formed macromolecules exhibit strong polymer-polymer interactions in aqueous milieu and therefore form thermoreversible physical hydrogels in pure water, physiological buffer or cell medium.
Len Ito et al.
FEBS letters, 585(3), 555-560 (2011-01-18)
Glycine amide (GlyAd), a typically amidated amino acid, is a versatile additive that suppresses protein aggregation during refolding, heat treatment, and crystallization. In spite of its effectiveness, the exact mechanism by which GlyAd suppresses protein aggregation remains to be elucidated.
Irene M Lagoja et al.
Chemistry & biodiversity, 1(1), 106-111 (2006-12-29)
Because of their easy availability and their relative chemical stability, urea, formic acid, and glycine might have played a role in the assembly process of nucleobases. In this paper, a short reaction path is described to prepare hypoxanthine starting from
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.