577944
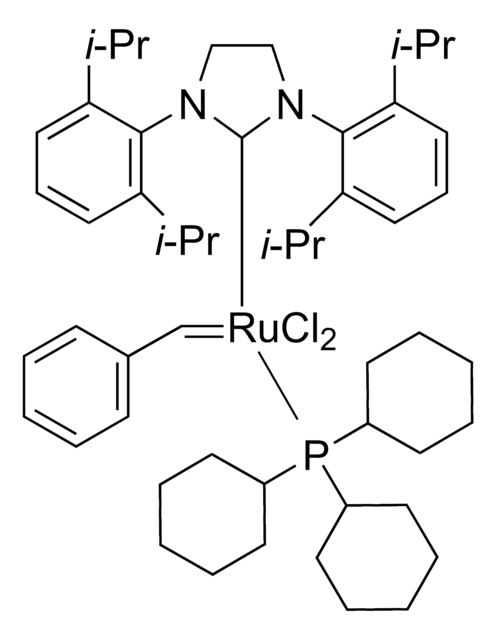
Hoveyda-Grubbs Catalyst® M700
Umicore
동의어(들):
Hoveyda-Grubbs Catalyst® 1st Generation, Hoveyda-Grubbs Catalyst® M70 (C601), Dichloro(2-isopropoxyphenylmethylene) (tricyclohexylphosphine)ruthenium(II), Dichloro(o-isopropoxyphenylmethylene)(tricyclohexylphosphine)ruthenium(II)
About This Item
추천 제품
Quality Level
형태
solid
반응 적합성
core: ruthenium
reagent type: catalyst
reaction type: Ring Opening Metathesis Polymerisation
mp
195-197 °C (lit.)
저장 온도
2-8°C
SMILES string
[H]\C(c1ccccc1OC(C)C)=[Ru](\Cl)Cl.C2CCC(CC2)P(C3CCCCC3)C4CCCCC4
InChI
1S/C18H33P.C10H12O.2ClH.Ru/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-8(2)11-10-7-5-4-6-9(10)3;;;/h16-18H,1-15H2;3-8H,1-2H3;2*1H;/q;;;;+2/p-2
InChI key
KMKCJXPECJFQPQ-UHFFFAOYSA-L
애플리케이션
Learn more about our metathesis catalysts
법적 정보
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
관련 콘텐츠
Olefin metathesis involves an organic reaction that redistributes fragments of alkenes (olefins) by cleavage and transformation of carbon-carbon double bonds.
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.









![Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene][[5-[(dimethylamino)sulfonyl]-2-(1-methylethoxy-O)phenyl]methylene-C]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/179/573/f48a2a1e-cf09-4151-8b78-2bab614efd5c/640/f48a2a1e-cf09-4151-8b78-2bab614efd5c.png)