모든 사진(1)
About This Item
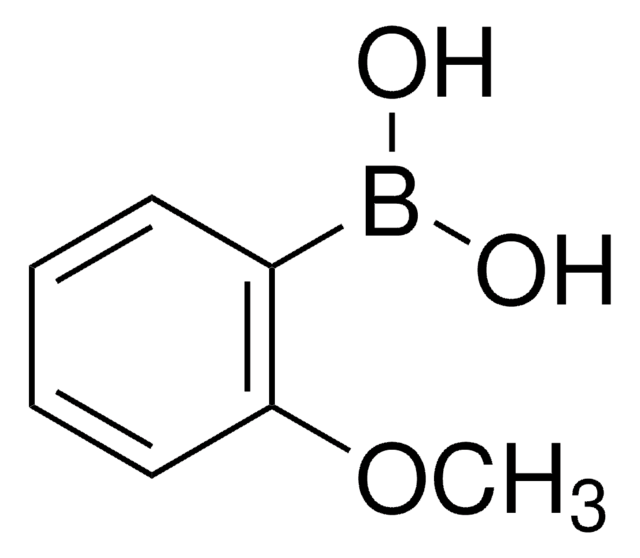
Linear Formula:
BrC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
215.04
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
mp
51-54 °C (lit.)
SMILES string
COc1ccc(C=O)cc1Br
InChI
1S/C8H7BrO2/c1-11-8-3-2-6(5-10)4-7(8)9/h2-5H,1H3
InChI key
QMPNFQLVIGPNEI-UHFFFAOYSA-N
일반 설명
3-Bromo-4-methoxybenzaldehyde is formed by the solvent-free bromination of 4-methoxybenzaldehyde using 1,3-di-n-butylimidazolium tribromide, as a brominating reagent.[1]
애플리케이션
3-Bromo-4-methoxybenzaldehyde may be used in the following studies:
- Asymmetric synthesis of a novel β-hydroxy-α-amino acid derivative, via Mukaiyama aldol reaction.[2]
- Synthesis of 2-(3-bromo-4-methoxyphenyl)-5-fluorobenzothiazole.[3]
- Preparation of 5-[(Z)-2-(3-bromo-4-methoxyphenyl)vinyl]-1,2-3-trimethoxybenzene.[4]
- Total synthesis of engelhardione.[5]
- Starting reagent for the synthesis of (2E)-3-(3-bromo-4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one.[6]
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
22287813
10th Internatl. Conf. on Organic Synthesis, Bangalore, India, December 1994 null
Li Shen et al.
Tetrahedron letters, 52(35), 4570-4574 (2011-09-20)
The total synthesis of the macrocyclic natural product engelhardione is reported. This effort led to the structural revision of the published structure of engelhardione to that of pterocarine. The revision reflects the change of the substitution pattern of one phenyl
Aromatic bromination of aldehydes and ketones using 1, 3-di-n-butylimidazolium tribromide [BBIm] Br3 ionic liquids under solvent-free conditions.
Borikar SP and Daniel T.
Journal of the Iranian Chemical Society, 8(2), 531-536 (2011)
(2E)-3-(3-Bromo-4-methoxyphenyl)-1-(4-methylphenyl)prop-2-en-1-one.
Dutkiewicz g, et al.
Acta Crystallographica Section E, Structure Reports Online, 67(4), 1024-1024 (2011)
Yali Kong et al.
Chemistry & biology, 12(9), 1007-1014 (2005-09-27)
Targeting the microtubule system represents an attractive strategy for the development of anticancer agents. In this study, we report a class of combretastatin A-4 (CA-4) analogs derivatized with a boronic acid moiety replacing the hydroxyl group on the C-ring of
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.