모든 사진(2)
About This Item
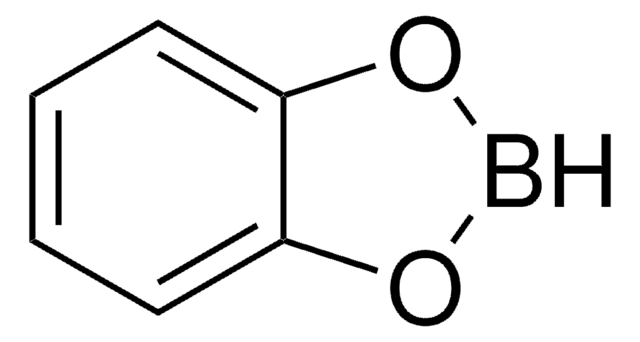
실험식(Hill 표기법):
C6H4BClO2
CAS Number:
Molecular Weight:
154.36
Beilstein:
2093985
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
형태
solid
mp
56-58 °C (lit.)
SMILES string
Clb1oc2ccccc2o1
InChI
1S/C6H4BClO2/c8-7-9-5-3-1-2-4-6(5)10-7/h1-4H
InChI key
AZYGEWXDKHFOKB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
B-Chlorocatecholborane is a boron reagent and a Lewis acid, known to facilitate the borylative cyclization of alkynes to yield the borylated heterocycles. It is also used in the preparation of lactones, and thiophenes.
애플리케이션
B-Chlorocatecholborane can be used:
- To prepare 2-arachidonoylglycerol by acetal cleavage of cis-arachidonoylbenzylidene glycerol.
- To prepare metal boryl complexes (Rh and Ir complexes) through oxidative addition.
- To remove the trityl group in one of the key steps for the synthesis of (−)-dictyostatin.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Sol. 1 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
140.0 °F - closed cup
Flash Point (°C)
60 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Chao Gao et al.
The Journal of organic chemistry, 85(16), 10350-10368 (2020-07-17)
In contrast to previously reported borylative heterocyclization methods, a reaction here proceeds without air-free techniques to access synthetically useful borylated thiophenes, benzothiophenes, and isocoumarins. A comparison of stability/decomposition rates in air of several catecholboronic ester (Bcat) compounds derived from different
Synthesis and biological evaluation of (−)-dictyostatin and stereoisomers
Shin Y, et al.
Tetrahedron, 63(35), 8537-8562 (2007)
Tetrahedron Letters, 26, 1411-1411 (1985)
Synthesis of rhodium and iridium boryl complexes via oxidative addition of haloboranes
Souza FES, et al.
Inorgorganica Chimica Acta, 358(5), 1501-1509 (2005)
Kang Yuan et al.
Organic letters, 19(6), 1462-1465 (2017-03-08)
We report here a facile B(C6F5)3 catalyzed trans-aminoboration of internal alkynes, furnishing 3-position borylated indoles at ambient temperature. This reaction proceeds with the breaking of a B-N bond and the formation of N-C and B-C bonds to produce indole and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.