217328
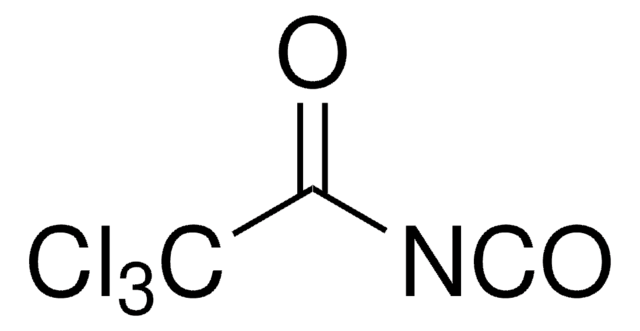
Trichloroacetyl isocyanate
96%
동의어(들):
2,2,2-Trichloroacetyl isocyanate, alpha,alpha,alpha-Trichloroacetyl isocyanate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
Cl3CCONCO
CAS Number:
Molecular Weight:
188.40
Beilstein:
971201
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
96%
refractive index
n20/D 1.480 (lit.)
bp
80-85 °C/20 mmHg (lit.)
density
1.581 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
ClC(Cl)(Cl)C(=O)N=C=O
InChI
1S/C3Cl3NO2/c4-3(5,6)2(9)7-1-8
InChI key
GRNOZCCBOFGDCL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Conformational stability and vibrational IR and Raman spectra of trichloroacetyl isocyanate has been investigated. Trichloroacetyl isocyanate is commonly employed as in situ derivatizing reagent for the 13C NMR studies of alcohols, phenols and amines. It undergoes 1,5-cycloaddition reaction with anhydro-2-deoxy-D-erythro- and -L-threo-pent-1-enitols and 1,5-anhydro-2-deoxy-D- and -L-arabino-hex-1-enitols to yield [2+2] and [4+2] cycloadducts.
애플리케이션
Trichloroacetyl isocyanate was used in catalytic one-pot dehydrative glycosylation of 1-hydroxy carbohydrate. It was also used as a reagent for the conversion of alcohols to carbamates†.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
150.8 °F - closed cup
Flash Point (°C)
66 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
NMR spectral studies-XII: Trichloroacetyl isocyanate as an in situ derivatizing reagent for 13C NMR spectroscopy of alcohols, phenols and amines.
Bose AK and Srinivasan PR.
Tetrahedron, 31(24), 3025-3029 (1975)
Hassan M Badawi et al.
Journal of molecular modeling, 8(2), 44-49 (2002-05-29)
The conformational stability and vibrational infrared and Raman spectra of trichloroacetyl isocyanate (CCl3CONCO) were investigated by ab initio MP2 and density functional B3LYP calculations using the 6-311++G** basis set. From the potential energy scans of the internal rotations in both
Tetrahedron Letters, 48, 65-65 (2007)
[2+ 2] Cycloaddition of trichloroacetyl isocyanate to glycals.
Chmielewski M and Kaluza Z.
Carbohydrate Research, 167, 143-152 (1987)
Tatsuya Shirahata et al.
Carbohydrate research, 345(6), 740-749 (2010-03-09)
Efficient catalytic and stereoselective glycosylation was achieved by activating a glycosyl N-trichloroacetylcarbamate with a catalytic amount of Lewis acid in the presence of a glycosyl acceptor and 5A molecular sieves. Catalytic one-pot dehydrative glycosylation of a 1-hydroxy carbohydrate was achieved
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.