おすすめの製品
詳細
Prolactin is a lactogenic hormone that plays a role in breast cancer, regulation of reproductive function, and immunoregulation. The prolactin cDNA encodes a 227 amino acid residue protein with a putative 28 amino residue signal peptide. Removal of the signal peptide results in the mature hormone corresponding to amino acids 29-227 of natural prolactin. There are several natural occurring molecular forms of prolactin, including a monomer, a non-glycosylated form, and a glycosylated form.
Prolactin is manufactured using an all-human production system, with full chemically defined ingredients and with no serum. It is therefore completely animal- and xeno-component free.
Research Area: IMMUNO AND CKS
Prolactin (PRL) is a multifunctional polypeptide hormone primarily produced by the lactotrophic cells of the anterior pituitary gland in vertebrates.
Prolactin (PRL) is a multifunctional polypeptide hormone primarily produced by the lactotrophic cells of the anterior pituitary gland in vertebrates.
アプリケーション
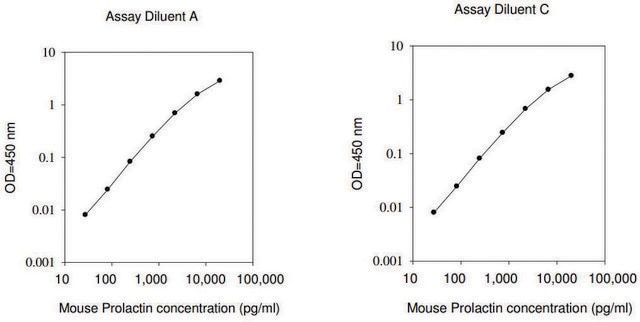
Prolactin human has been used:
- in in vitro experiments to examine its effects in sleep-like concentrations on T-cell migration
- to study its effects on claudin 2 (CLDN2) expression in the Caco-2 intestinal epithelial cell model
- in microplate assays to demonstrate the specificity of the antibodies for vasoinhibin
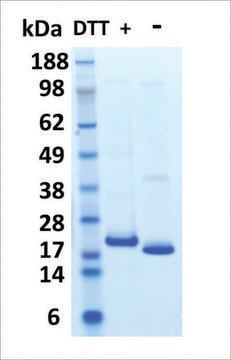
Prolactin is glycosylated.
生物化学的/生理学的作用
Glycosylated human prolactin (G-hPRL) was first isolated and purified from human pituitaries by Lewis et al., with an estimated molecular mass of 25,000 Da and an immunological and biological activity of 25–50% that of non-glycosylated hPRL. The presence of a unique and partially occupied glycosylation site in Asn-31 in human, monkey, ovine, porcine, dromedary, equine and whale PRL makes it an ideal model of glycosylation for N-glycan studies since it exhibits the simplest type of glycosylation macroheterogeneity, with an occupancy range of 10-30% of G-hPRL relative to the total hPRL of either pituitary or recombinant origin. It has been postulated that hPRL glycosylation might possibly modulate the bioactivity of the circulating pool of the hormone, perhaps by selectively down regulating PRL action at individual target tissues.
Prolactin is recognized in breast milk and can enhance Ca2+ absorption through both transcellular and paracellular pathways in the small and large intestine. It is crucial for lactation and reproduction and is demonstrated to have numerous effects on growth, development, metabolism, immunoregulation, and protection. The prolactin signaling pathway begins with the binding of prolactin to the prolactin receptor (PRLR).
物理的形状
This product is supplied as a solution in 0.2 μm filtered phosphate buffered saline with no additives or carrier proteins. It is aseptically filled.
調製ノート
Briefly centrifuge the vial before opening. After initial thawing it is recommended to store the protein in working aliquots at -20°C. The product can be diluted in PBS.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Repr. 1B
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SRP9000-50UG:
SRP9000-VAR:
SRP9000-50UG-PW:
SRP9000-BULK:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
U J Lewis et al.
Endocrinology, 124(3), 1558-1563 (1989-03-01)
Two forms of glycosylated PRL (G-PRL) which differed in their binding properties to Concanavalin-A (Con-A) were isolated from human pituitary glands. One form, G1-hPRL, was only slightly retarded by Con-A; the other, G2-hPRL, was adsorbed by Con-A and could be
V Y Butnev et al.
Journal of protein chemistry, 15(5), 413-426 (1996-07-01)
Glycosylated equine prolactin (G-ePRL) and nonglycosylated ePRL were purified to homogeneity from side fractions obtained during isolation of LH/FSH from horse pituitaries. Both PRL forms were isolated together in high yield by the isolation procedure used for glycosylated porcine PRL/(G-pPRL)
Structural variants of prolactin: occurrence and physiological significance.
Y N Sinha
Endocrine reviews, 16(3), 354-369 (1995-06-01)
T Hoffmann et al.
Journal of endocrinological investigation, 16(10), 807-816 (1993-11-01)
To analyze the role of individual glycosylation pattern on PRL biopotency, monomeric prolactin (PRL), secreted by human prolactinoma cells in culture, was isolated by gel filtration and separated by affinity chromatography on Concanavalin A-Sepharose or Lentil-Agarose. These lectins allowed the
M E Freeman et al.
Physiological reviews, 80(4), 1523-1631 (2000-10-04)
Prolactin is a protein hormone of the anterior pituitary gland that was originally named for its ability to promote lactation in response to the suckling stimulus of hungry young mammals. We now know that prolactin is not as simple as
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)