おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
形状
powder
光学活性
[α]/D +95 to +115°, c = 1 in H2O
薬剤管理
regulated under CDSA - not available from Sigma-Aldrich Canada
保管条件
desiccated
色
white to beige
溶解性
H2O: 25 mg/mL, clear
保管温度
2-8°C
SMILES記法
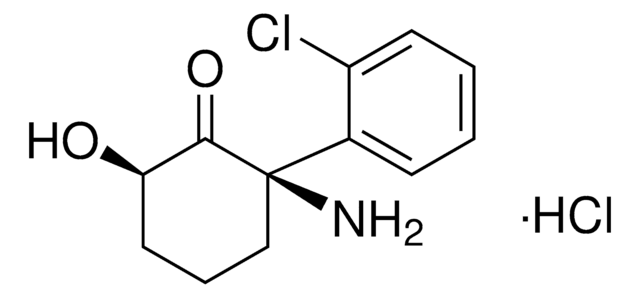
[H]Cl.ClC1=CC=CC=C1[C@](CCC[C@@H]2O)(N)C2=O
生物化学的/生理学的作用
(2S,6S)-Hydroxynorketamine hydrochlorideKetamine is a metabolite associated with increased locomotor activity and motor incoordination.
It has been found that the NMDAR antagonist (R,S)-ketamine must be metabolized to (2S,6S;2R,6R)-hydroxynorketamine (HNK) to have antidepressant effects. The (2R,6R)-HNK enantiomer appears to be the enantiomer most responsible for antidepressant effects, while (2S,6S)-hydroxynorketamine was associated with increased locomotor activity and motor incoordination. (2S,6S)-HNK, was also found to increase the function of the mammalian target of rapamycin (mTOR) 2-fold at 0.05 nM. (2S,6S)-HNK and (2R,6R)-HNK both inhibited intracellular concentrations of D-serine, an endogenous NMDA receptor co-agonist, with IC50 values of 0.18 nM and 0.68 nM, respectively. Neither bind with high affinity to NMDA receptors, with Ki values of 21.19 μM and > 100 μM for (2S,6S)-HNK and (2R,6R)-HNK, respectively.
It has been found that the NMDAR antagonist (R,S)-ketamine must be metabolized to (2S,6S;2R,6R)-hydroxynorketamine (HNK) to have antidepressant effects. The (2R,6R)-HNK enantiomer appears to be the enantiomer most responsible for antidepressant effects, while (2S,6S)-hydroxynorketamine was associated with increased locomotor activity and motor incoordination. (2S,6S)-HNK, was also found to increase the function of the mammalian target of rapamycin (mTOR) 2-fold at 0.05 nM. (2S,6S)-HNK and (2R,6R)-HNK both inhibited intracellular concentrations of D-serine, an endogenous NMDA receptor co-agonist, with IC50 values of 0.18 nM and 0.68 nM, respectively. Neither bind with high affinity to NMDA receptors, with Ki values of 21.19 μM and > 100 μM for (2S,6S)-HNK and (2R,6R)-HNK, respectively.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML1875-BULK:
SML1875-5MG:
SML1875-BULK-CR:
SML1875-VAR:
SML1875-25MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Panos Zanos et al.
Nature, 533(7604), 481-486 (2016-05-05)
Major depressive disorder affects around 16 per cent of the world population at some point in their lives. Despite the availability of numerous monoaminergic-based antidepressants, most patients require several weeks, if not months, to respond to these treatments, and many
Nagendra S Singh et al.
PloS one, 11(4), e0149499-e0149499 (2016-04-21)
D-Serine is an endogenous NMDA receptor co-agonist that activates synaptic NMDA receptors modulating neuronal networks in the cerebral cortex and plays a key role in long-term potentiation of synaptic transmission. D-serine is associated with NMDA receptor neurotoxicity and neurodegeneration and
Rajib K Paul et al.
Anesthesiology, 121(1), 149-159 (2014-06-18)
Subanesthetic doses of (R,S)-ketamine are used in the treatment of neuropathic pain and depression. In the rat, the antidepressant effects of (R,S)-ketamine are associated with increased activity and function of mammalian target of rapamycin (mTOR); however, (R,S)-ketamine is extensively metabolized
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)