おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to BP 629
traceable to Ph. Eur. Y0001080
traceable to USP 1379401
APIファミリー
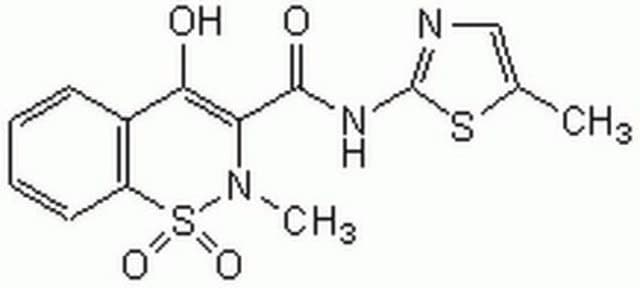
meloxicam
CofA
current certificate can be downloaded
包装
pkg of 500 mg
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
InChI
1S/C14H13N3O4S2/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2/h3-7,19H,1-2H3,(H,15,16)/b13-11+
InChI Key
DWMREKMVXIFPFM-ACCUITESSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Meloxicam is an oxicam derivative and belongs to the group of non-steroidal anti-inflammatory drugs (NSAID), used for its analgesic, anti-inflammatory, and antipyretic properties. It acts by inhibiting the activity of prostaglandin synthetase, thereby preventing the synthesis of prostaglandins and decreasing inflammation.
アプリケーション
- Development of three spectrophotometric methods to determine meloxicam in its pharmaceutical formulations
- Voltammetric determination of meloxicam in pharmaceutical formulations using a boron-doped diamond electrode (BDDE)
- High-performance liquid chromatography-based quantitative analysis of meloxicam in its different tablet formulations
- Development and validation of a reversed-phase HPLC method for the separation and estimation of meloxicam in its pharmaceutical dosage forms
- Quantification of meloxicam and piroxicam from plasma samples using a liquid chromatography method combined with UV detection
アナリシスノート
脚注
おすすめ製品
関連製品
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1799-500MG-PW:
PHR1799-500MG:
Choose from one of the most recent versions:
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)