PHR1302
Pramoxine hydrochloride
Pharmaceutical Secondary Standard; Certified Reference Material
別名:
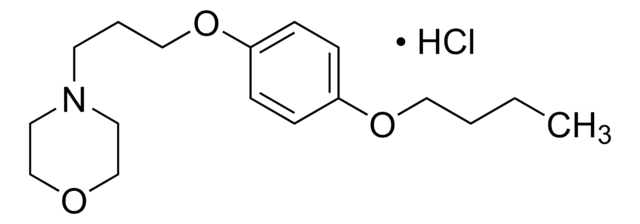
Pramoxine hydrochloride, 4-[3-(4-Butoxyphenoxy)propyl]morpholine hydrochloride
About This Item
おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to USP 1554002
APIファミリー
pramoxine
CofA
current certificate can be downloaded
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-30°C
SMILES記法
Cl.CCCCOc1ccc(OCCCN2CCOCC2)cc1
InChI
1S/C17H27NO3.ClH/c1-2-3-12-20-16-5-7-17(8-6-16)21-13-4-9-18-10-14-19-15-11-18;/h5-8H,2-4,9-15H2,1H3;1H
InChI Key
SYCBXBCPLUFJID-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
アプリケーション
生物化学的/生理学的作用
アナリシスノート
その他情報
脚注
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1302-1G:
PHR1302-1G-PW:
Choose from one of the most recent versions:
試験成績書(COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)