SCR544
Human STEMCCA Constitutive Polycistronic (OKSM) Lentivirus Reprogramming Kit
The Human STEMCCA Constitutive Polycistronic Lentivirus Kit contains high titer polycistronic lentivirus & Polybrene transfection reagent that have been validated for the generation of human iPS cells from human foreskin fibroblasts.
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
UNSPSCコード:
12352207
eCl@ss:
32161000
NACRES:
NA.71
おすすめの製品
品質水準
メーカー/製品名
STEMCCA
テクニック
cell culture | stem cell: suitable
入力
sample type induced pluripotent stem cell(s)
輸送温度
dry ice
詳細
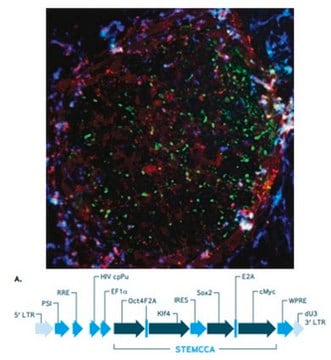
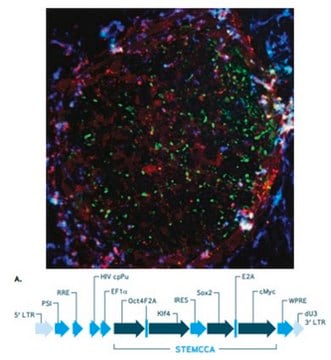
The Human STEMCCA Constitutive Polycistronic (OKSM) Lentivirus Kit contains high titer polycistronic (OKSM) lentivirus and Polybrene transfection reagent that have been validated for the generation of human induced pluripotent stem cells from human foreskin fibroblasts (HFFs). The STEMCCA vector is comprised of the transcription factors human Oct-4, Klf4, SOX-2, and c-Myc (OKSM), separated by the self-cleaving 2A peptide and IRES sequences (Sommer CA, 2009). The use of a single polycistronic lentiviral vector significantly improves reprogramming efficiencies and reduces the number of viral integrations.
構成
1. Human STEMCCA Constitutive (OKSM) Lentivirus: (Part number SCR544-1) Two (2) vials of EF1α-hSTEMCCA (OKSM) Lentivirus (Part number CS204502). Each vial contains 15 µL of high titer lentivirus.
2. Polybrene Transfection Reagent: (Part number TR-1003-50UL) One (1) vial containing 50 µL of 10 mg/mL stock.
2. Polybrene Transfection Reagent: (Part number TR-1003-50UL) One (1) vial containing 50 µL of 10 mg/mL stock.
品質
Tested to confirm the generation of iPS cells from p6 human foreskin fibroblasts. Other cell types have not been tested and thus similar results can not be guaranteed.
保管分類コード
12 - Non Combustible Liquids
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SCR544:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Yang Yu et al.
Scientific reports, 5, 10114-10114 (2015-05-13)
Human pluripotent stem cells, including cloned embryonic and induced pluripotent stem cells, offer a limitless cellular source for regenerative medicine. However, their derivation efficiency is limited, and a large proportion of cells are arrested during reprogramming. In the current study
Frank Griscelli et al.
Oncotarget, 10(28), 2693-2708 (2019-05-21)
Recent development of cell reprogramming technologies brought a major hope for future cell therapy applications by the use of these cells or their derivatives. For this purpose, one of the major requirements is the absence of genomic alterations generating a
Jun-Quan Li et al.
Molecular medicine reports, 9(3), 837-842 (2014-01-09)
Myocardial infarction (MI) is an increasing medical problem; however, its pathogenesis has yet to be elucidated and more effective treatment strategies are required. Induced pluripotent stem cells (iPSCs) were recently successfully generated using human somatic cells transfected with four transcription
Martin Madill et al.
Molecular brain, 10(1), 22-22 (2017-06-15)
Amyotrophic lateral sclerosis, a devastating neurodegenerative disease, is characterized by the progressive loss of motor neurons and the accumulation of misfolded protein aggregates. The latter suggests impaired proteostasis may be a key factor in disease pathogenesis, though the underlying mechanisms
Areechun Sotthibundhu et al.
Stem cell research & therapy, 7(1), 166-166 (2016-11-17)
Cellular reprogramming is a stressful process, which requires cells to engulf somatic features and produce and maintain stemness machineries. Autophagy is a process to degrade unwanted proteins and is required for the derivation of induced pluripotent stem cells (iPSCs). However
資料
Fibroblast growth factors (FGFs) regulate developmental pathways and mesoderm/ectoderm patterning in early embryonic development.
プロトコル
Stem cell reprogramming protocols to generate human induced pluripotent stem cells (iPSCs) including viral and non-viral RNA based methods.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)