420201
JMJD2 Inhibitor, 5-carboxy-8HQ
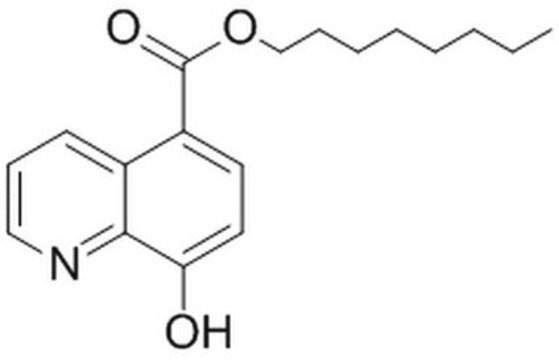
The JMJD2 Inhibitor, 5-carboxy-8HQ, also referenced under CAS 5852-78-8, controls the biological activity of JMJD2. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
別名:
JMJD2 Inhibitor, 5-carboxy-8HQ, JHDM Inhibitor I, Histone Lysine Demethylase Inhibitor II, 5-carboxy-8HQ, IOX1
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
おすすめの製品
品質水準
アッセイ
≥95% (HPLC)
形状
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
protect from light
色
orange
溶解性
DMSO: 50 mg/mL
輸送温度
ambient
保管温度
2-8°C
InChI
1S/C10H7NO3/c12-8-4-3-7(10(13)14)6-2-1-5-11-9(6)8/h1-5,12H,(H,13,14)
InChI Key
JGRPKOGHYBAVMW-UHFFFAOYSA-N
詳細
A cell-permeable, 5-carboxy-8-hydroxyquinoline that acts as a 2-oxoglutarate competitive inhibitor of JMJD (IC50 = 200 nM against JMJD2E in a FDH coupled assay). It demonstrates higher selectivity than 2.4-PDCA against other 2-OG oxygenases (IC50 = 2.4 µM, 1.7 µM, 20.5 µM, and 14.3 µM for JMJD2E, JMJD2A, FIH, and PHD2, respectively, in a MALDI-TOF MS assay). Additionally, it is shown to inhibit H3K9me3 demethylation (IC50 = 86.5 µM) in JMJD2A-transfected HeLa cells in a cellular demethylase assay, dose-dependently.
A cell-permeable, 5-carboxy-8-hydroxyquinoline that acts as a 2-oxoglutarate competitive inhibitor of JMJD (IC50 = 200 nM against JMJD2E in a FDH coupled assay). It demonstrates higher selectivity than 2.4-PDCA against other 2-OG oxygenases (IC50 = 2.4 µM, 1.7 µM, 20.5 µM, and 14.3 µM for JMJD2E, JMJD2A, FIH, and PHD2, respectively, in a MALDI-TOF MS assay). Additionally, it is shown to inhibit H3K9me3 demethylation (IC50 = 86.5 µM) in JMJD2A-transfected HeLa cells in a cellular demethylase assay, dose-dependently.
This probe is supplied in conjunction with the Structural Genomics Consortium (SGC). For further characterization details, please visit the IOX1 probe summary on the SGC website.
This probe is supplied in conjunction with the Structural Genomics Consortium (SGC). For further characterization details, please visit the IOX1 probe summary on the SGC website.
包装
Packaged under inert gas
警告
Toxicity: Regulatory Review (Z)
再構成
Following reconstiution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
その他情報
Hamada, S., et al. 2010. J. Med. Chem.53, 5629.
King, D.N.F., et al. 2010. PLoS One5, e15535.
King, D.N.F., et al. 2010. PLoS One5, e15535.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
420201-MG:
420201-5MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Shohei Hamada et al.
Journal of medicinal chemistry, 53(15), 5629-5638 (2010-08-06)
Selective inhibitors of Jumonji domain-containing protein (JMJD) histone demethylases are candidate anticancer agents as well as potential tools for elucidating the biological functions of JMJDs. On the basis of the crystal structure of JMJD2A and a homology model of JMJD2C
Oliver N F King et al.
PloS one, 5(11), e15535-e15535 (2010-12-03)
Small molecule modulators of epigenetic processes are currently sought as basic probes for biochemical mechanisms, and as starting points for development of therapeutic agents. N(ε)-Methylation of lysine residues on histone tails is one of a number of post-translational modifications that
Ignacio Campillo-Marcos et al.
Frontiers in cell and developmental biology, 9, 715126-715126 (2021-09-21)
Chromatin is dynamically remodeled to adapt to all DNA-related processes, including DNA damage responses (DDR). This adaptation requires DNA and histone epigenetic modifications, which are mediated by several types of enzymes; among them are lysine methyltransferases (KMTs). KMT inhibitors, chaetocin
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)