おすすめの製品
形状
methanol solution
包装
pkg of 1 × 1 mL (LM1003-1EA)
メーカー/製品名
Avanti Research™ - A Croda Brand LM1003
濃度
~10 μg/mL (Refer to C of A for lot specific concentration.)
輸送温度
dry ice
保管温度
−20°C
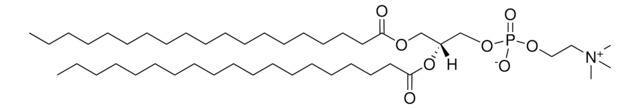
SMILES記法
[O-]P(OCC[N+](C)(C)C)(OC[C@]([H])(OC(CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CC)=O)COC(CCCCCCCCCCCCCCCCCCCC)=O)=O
関連するカテゴリー
詳細
Phosphatidylcholine (PC) is an abundant phospholipid in the pulmonary surfactant.
アプリケーション
21:0-22:6 PC or 1-heneicosanoyl-2-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycero-3-phosphocholine has been used as a lipid standard/LIPID MAPS (LM) internal standard for phospholipid quantification/analysis using mass spectrometry (MS) and liquid chromatography (LC)-MS.
包装
2 mL Amber Glass Sealed Ampule (LM1003-1EA)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
ターゲットの組織
Eyes
保管分類コード
3 - Flammable liquids
WGK
WGK 2
引火点(°F)
49.5 °F - closed cup
引火点(℃)
9.7 °C - closed cup
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Per Larsson et al.
Respiratory physiology & neurobiology, 243, 39-46 (2017-05-16)
Exhaled particles constitute a micro-sample of respiratory tract lining fluid. Inhalations from low lung volumes generate particles in small airways by the airway re-opening mechanism. Forced exhalations are assumed to generate particles in central airways by mechanisms associated with high
Penelope Dimas et al.
eLife, 8 (2019-05-08)
Oligodendrocytes (OLs) support neurons and signal transmission in the central nervous system (CNS) by enwrapping axons with myelin, a lipid-rich membrane structure. We addressed the significance of fatty acid (FA) synthesis in OLs by depleting FA synthase (FASN) from OL
Laura Montani et al.
The Journal of cell biology, 217(4), 1353-1368 (2018-02-13)
Myelination calls for a remarkable surge in cell metabolism to facilitate lipid and membrane production. Endogenous fatty acid (FA) synthesis represents a potentially critical process in myelinating glia. Using genetically modified mice, we show that Schwann cell (SC) intrinsic activity
Rachel Fickes et al.
Rapid communications in mass spectrometry : RCM, 30(24), 2601-2606 (2016-10-01)
Structural analogs of the bioactive lipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol were synthesized with a xylitol polar head group and both diacyl and diether radyl groups. Mass spectral characterization of xylitol phospholipids (PX) was carried out using collisional activation and high-resolution mass measurements of
Ulrike Bruning et al.
Cell metabolism, 28(6), 866-880 (2018-08-28)
The role of fatty acid synthesis in endothelial cells (ECs) remains incompletely characterized. We report that fatty acid synthase knockdown (FASNKD) in ECs impedes vessel sprouting by reducing proliferation. Endothelial loss of FASN impaired angiogenesis in vivo, while FASN blockade reduced
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)