927678
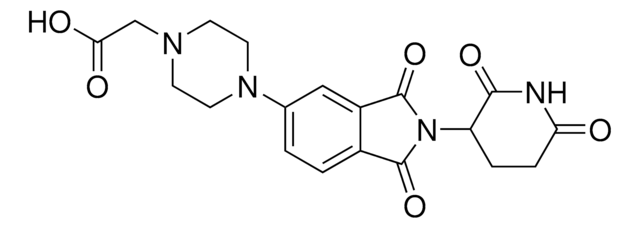
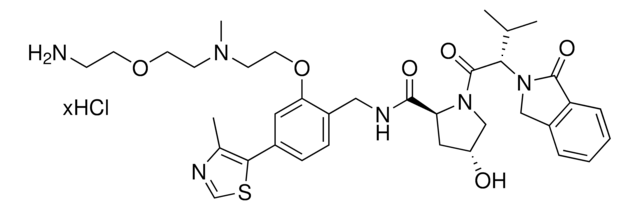
Pomalidomide-difluoroPEG1-C4-piperazine Hydrochloride
≥95%
別名:
2-(2,2-Difluoro-3-(4-(piperazin-1-yl)butoxy)propoxy)-N-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)acetamide hydrochloride
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C26H33F2N5O7 · xHCl
分子量:
565.57 (free base basis)
UNSPSCコード:
12352101
NACRES:
NA.21
おすすめの製品
ligand
pomalidomide
品質水準
アッセイ
≥95%
形状
powder
保管温度
2-8°C
SMILES記法
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(COCC(F)(F)COCCCCN4CCNCC4)=O)=O)NC1=O.Cl
アプリケーション
Protein degrader building block Pomalidomide-difluoroPEG1-C4-piperazine Hydrochloride enables the synthesis of molecules for targeted protein degradation and PROTAC (proteolysis-targeting chimeras) technology. This conjugate contains a Cereblon (CRBN)-recruiting ligand, a fluorinated linker with both hydrophobic and hydrophilic moieties, and a pendant amine for reactivity with a carboxylic acid on the target ligand. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and PROTAC, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a pendant amine, parallel synthesis can be used to more quickly generate PROTAC libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
Targeted Protein Degradation
Targeted Protein Degradation
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Repr. 1B
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
927678-50MG:
927678-BULK:
927678-VAR:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Kedra Cyrus et al.
Molecular bioSystems, 7(2), 359-364 (2010-10-06)
Conventional genetic approaches have provided a powerful tool in the study of proteins. However, these techniques often preclude selective manipulation of temporal and spatial protein functions, which is crucial for the investigation of dynamic cellular processes. To overcome these limitations
Philipp M Cromm et al.
Cell chemical biology, 24(9), 1181-1190 (2017-06-27)
Traditional pharmaceutical drug discovery is almost exclusively focused on directly controlling protein activity to cure diseases. Modulators of protein activity, especially inhibitors, are developed and applied at high concentration to achieve maximal effects. Thereby, reduced bioavailability and off-target effects can
Momar Toure et al.
Angewandte Chemie (International ed. in English), 55(6), 1966-1973 (2016-01-13)
The current inhibitor-based approach to therapeutics has inherent limitations owing to its occupancy-based model: 1) there is a need to maintain high systemic exposure to ensure sufficient in vivo inhibition, 2) high in vivo concentrations bring potential for off-target side effects, and 3) there is
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)