917184
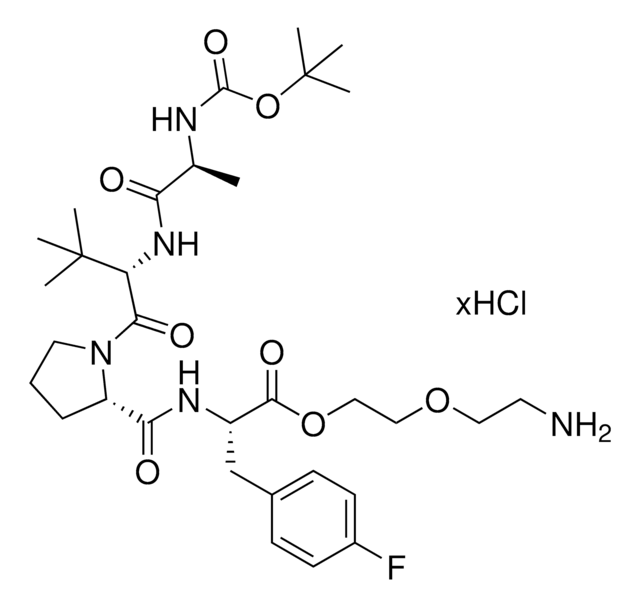
BocA1V1PF2-OC10-NH2 hydrochloride
別名:
10-Aminodecyl (S)-2-((S)-1-((S)-2-((S)-2-((tert-butoxycarbonyl)amino)propanamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxamido)-3-(4-fluorophenyl)propanoate hydrochloride, AVP conjugate for IAP-mediated protein degrader development, SNIPER building block
About This Item
おすすめの製品
ligand
BocA1V1PF2
品質水準
形状
powder
反応適合性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能基
amine
保管温度
2-8°C
SMILES記法
C[C@H](NC(OC(C)(C)C)=O)C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(OCCCCCCCCCCN)=O)CC2=CC=C(C=C2)F)=O)=O)C(C)(C)C)=O.Cl
アプリケーション
Developed in partnership with ComInnex, this conjugate contains an in silico-derived IAP-recruiting ligand, an alkyl-chain crosslinker, and a pendant amine for reactivity with an acid on a target warhead. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and protein degrader, many analogs are prepared to screen for optimal target degradation. When used with other When used with other protein degrader building blocks with a terminal amine, including CRBN and VHL targeted, parallel synthesis can be used to more quickly generate SNIPER and PROTAC® degrader libraries that feature variation in crosslinker length, composition, and E3 ligase ligand. Learn more about the novel IAP ligands generated through virtual screening of AVP mimetics in our Technology Spotlight.
Building blocks in this series:
917478 BocA1V1PF2
916927 BocA1V1PF2-OC6-NH2 hydrochloride
917184 BocA1V1PF2-OC10-NH2 hydrochloride
917435 BocA1V1PF2-OPEG1-NH2 hydrochloride
917680 BocA1V1PF2-OPEG3-NH2 hydrochloride
その他情報
法的情報
関連製品
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
917184-BULK:
917184-VAR:
917184-50MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
資料
Plate of 80 ligands against E3 ligase IAP designed by ComInnex; allows creation of bifunctional targeted protein degraders or molecular glues.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)