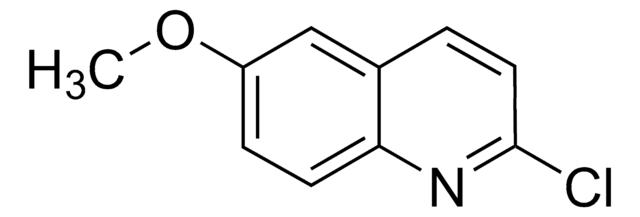
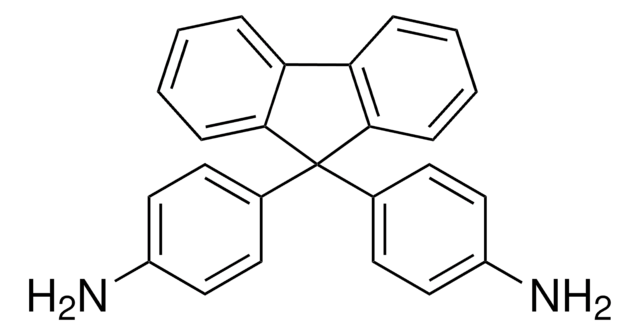
おすすめの製品
詳細
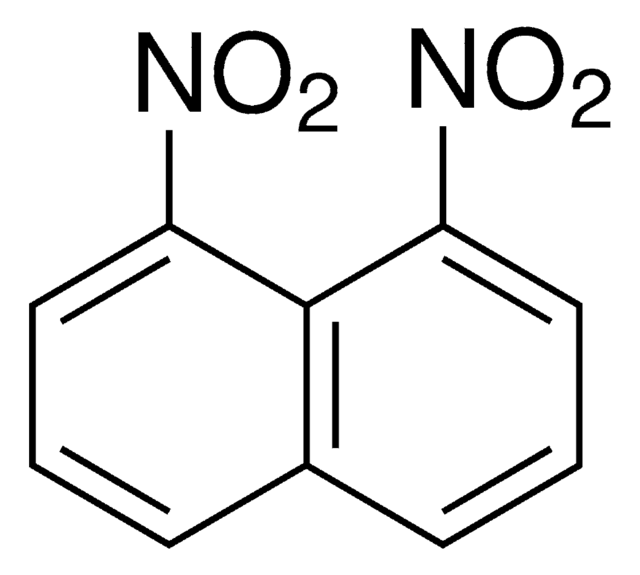
1,3-Dinitronaphthalene, 1,5-dinitronaphthalene and 1,8-dinitronaphthalene are the three dinitronaphthalene isomers. Their photocatalytic oxidation reactions in the presence of TiO2 Degussa P-25 grade has been investigated.[1] 1,5-Dinitronaphthalene has been reported as environmental pollutant. Its genotoxic effects during in vivo wing somatic mutation and recombination test (SMART) of Drosophila melanogaster has been investigated.[2] Delocalized intervalence radical anions of 1,5-dinitronaphthalene exhibits charge-transfer bands in their near-IR spectra.[3] Crystals of 1,5-dinitronaphthalene are reported to be monoclinic with two molecules in each unit cell.[4]
アプリケーション
1,5-Dinitronaphthalene has been employed as standard for the analysis of compounds of environmental interest by new nano-high-performance liquid chromatography-electron ionization mass spectrometry.[5]
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Aquatic Chronic 3 - Eye Dam. 1 - Muta. 2 - Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第5類:自己反応性物質
ニトロ化合物
危険等級I
第一種自己反応性物質
Jan Code
42135-VAR:
42135-BULK:
42135-100G:
Kei Miyazawa et al.
Journal of mass spectrometry : JMS, 55(12), e4668-e4668 (2020-11-03)
Fragmentation of peptide radical cations [M]. + has been examined using matrix-assisted laser desorption/ionization (MALDI) in-source decay (ISD) with hydrogen-abstracting nitro-substituted matrices. The ISD spectra of peptides containing an arginine (Arg) residue at carboxyl (C)-termini showed preferential [w]+ ions when
Miray Bekbolet et al.
Chemosphere, 75(8), 1008-1014 (2009-02-24)
A combination of photocatalytic oxidation experiments and quantum mechanical calculations was used in order to describe the mechanism and the nature of the photocatalytic oxidation reactions of dinitronaphthalane isomers and interprete their reactivities within the framework of the Density Functional
Nano-high-performance liquid chromatography-electron ionization mass spectrometry approach for environmental analysis.
Cappiello A, et al.
Analytica Chimica Acta, 493(2), 125-136 (2003)
Yuki Hiruta et al.
The Analyst, 141(3), 910-917 (2015-12-10)
Temperature-responsive polymers incorporating molecular-recognition sites were developed as stationary phases for high-performance liquid chromatography (HPLC). The grafted stationary phases consisted of functional copolymers composed of N-isopropylacrylamide (NIPAAm) and N-acryloyl aromatic amino acid methyl esters, i.e., phenylalanine and tryptophan methyl esters
The crystal structure of 1, 5-dinitronaphthalene.
Trotter J.
Acta Crystallographica, 13(2), 95-99 (1960)
アクティブなフィルタ
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)