おすすめの製品
グレード
technical grade
アッセイ
95%
形状
liquid
屈折率
n20/D 1.484 (lit.)
bp
139-141 °C/60 mmHg (lit.)
密度
1.69 g/mL at 25 °C (lit.)
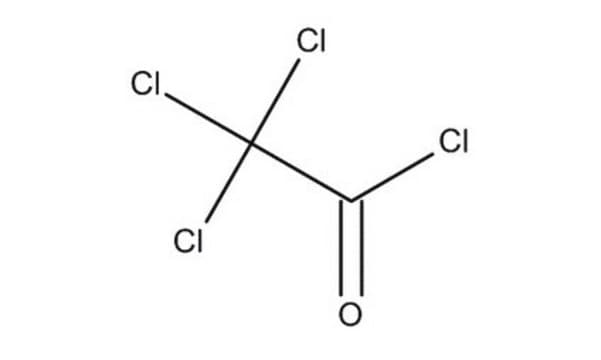
SMILES記法
ClC(Cl)(Cl)C(=O)OC(=O)C(Cl)(Cl)Cl
InChI
1S/C4Cl6O3/c5-3(6,7)1(11)13-2(12)4(8,9)10
InChI Key
MEFKFJOEVLUFAY-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
Trichloroacetic anhydride is reported to be an efficient derivatization reagent, since it produces stable derivatives having both a higher mass fragmentation pattern and lesser volatility than derivatives of trifluoroacetic anhydride (TFAA) or heptafluorobutyric anhydride (HFBA). The interaction of thymidine 5′-phosphate with trichloroacetic anhydride in dimethylformamide and acetonitrile in the presence of tertiary amines hs been studied.
アプリケーション
Trichloroacetic anhydride may be used as derivatization reagent for the following studies:
- Simultaneous analysis of amphetamine, methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA or Ecstasy) in urine samples by solid-phase extraction, derivatization and gas chromatography/mass spectrometry.
- Analysis of halogenated amphetamines in brain tissue from rats by gas chromatography with electron-capture detection (GC-ECD).
- Quantitative determination of the metabolites of l-alpha-acetylmethadol (LAAM), such as noracetylmethadol, dinoracetylmethadol, methadol and normethadol by electron capture gas-liquid chromatography.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Skin Corr. 1A
保管分類コード
8A - Combustible corrosive hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
367710-100G:
367710-25G:
367710-VAR:
367710-BULK:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
R J Czarny et al.
Journal of analytical toxicology, 13(5), 257-262 (1989-09-01)
An analytical procedure for methamphetamine and amphetamine has been developed using 4-carbethoxyhexafluorobutyryl chloride. This derivatizing reagent has all the previously reported advantages of trichloroacetic anhydride (TCAA) in that it produces stable derivatives having both a higher mass fragmentation pattern and
V S Bogachev et al.
Bioorganicheskaia khimiia, 29(1), 64-74 (2003-03-28)
The interaction of thymidine 5'-phosphate with trichloroacetic anhydride, trichloroacetyl chloride, and tribromoacetyl bromide was studied in dimethylformamide and acetonitrile in the presence of tertiary amines. The first two reactions gave the mixed anhydride of trichloroacetic and thymidylic acids (acyl phosphate)
B K Gan et al.
Journal of forensic sciences, 36(5), 1331-1341 (1991-09-01)
A rapid and effective solid-phase extraction procedure using Bond Elute Certify bonded silica sorbent cartridges was adopted to extract amphetamine, methamphetamine, and 3,4-methylenedioxymethamphetamine (MDMA or Ecstasy) from urine samples. The extract was derivatized with trichloroacetic anhydride prior to gas chromatography/mass
D H Lau et al.
Journal of chromatography, 129, 329-338 (1976-12-22)
Six reagents-trichloroacetyl chloride, trichloroacetic anhydride, pentafluorobenzoyl chloride, heptafluorobutyryl chloride, heptafluorobutyric anhydride, and trifluoroacetic anhydride- were evaluated as potential derivatizing reagents for quantitating the metabolites of l-alpha-acetylmethadol (LAAM) -noracetylmethadol, dinoracetylmethadol, methadol, and normethadol-by electron capture gas-liquid chromatography. All of the reagents
M L Owen et al.
Journal of pharmacological methods, 25(2), 147-155 (1991-04-01)
p-Chloro-alpha-fluoromethylphenylethylamine (fluoro-p-chloroamphetamine) (FpCA) has been shown in acute studies to be a less potent depletor of the neurotransmitter amine 5-hydroxytryptamine (5-HT) in brain than is p-chloroamphetamine (pCA). Gas chromatographic assay procedures for FpCA and PCA have been developed in our
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)