1A00050
USP
Rosuvastatin Ketone
Pharmaceutical Analytical Impurity (PAI)
Sinonimo/i:
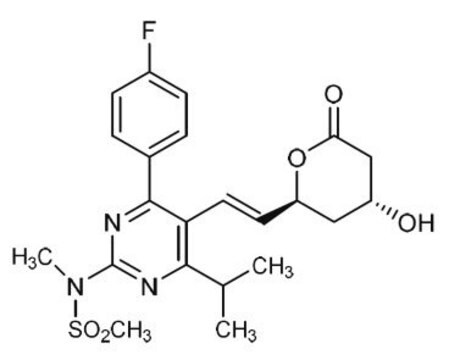
(3R,6E)-7-[4-(4-Fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoic acid, (R,E)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-yl)-3-hydroxy-5-oxohept-6-enoic acid, (3R,6E)-7-[4-(4-Fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoic acid, 5-Oxorosuvastatin
About This Item
Prodotti consigliati
Grado
pharmaceutical analytical impurity (PAI)
agenzia
USP
Famiglia di API
rosuvastatin
Produttore/marchio commerciale
USP
applicazioni
pharmaceutical
Formato
neat
Temperatura di conservazione
2-8°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Rosuvastatin Calcium
Therapeutic Area: Antihyperlipidemics
For more information about this PAI, visit here.
Applicazioni
Caratteristiche e vantaggi
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Risultati analitici
Altre note
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Contenuto correlato
Un ampio assortimento di standard di riferimento primari altamente caratterizzati da utilizzare seguendo i metodi monografici di USP-NF per l’analisi di principi attivi e forme di dosaggio, di eccipienti farmaceutici, ingredienti e integratori alimentari.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.