SMB00609
6,6′-Dihydroxythiobinupharidine
≥95% (HPLC)
Sinonimo/i:
6,6′-Dihydroxythionuphlutine A, Nuphleine
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥95% (HPLC)
Stato
powder
Spettro attività antibiotica
Gram-positive bacteria
Modalità d’azione
enzyme | inhibits
Temperatura di conservazione
−20°C
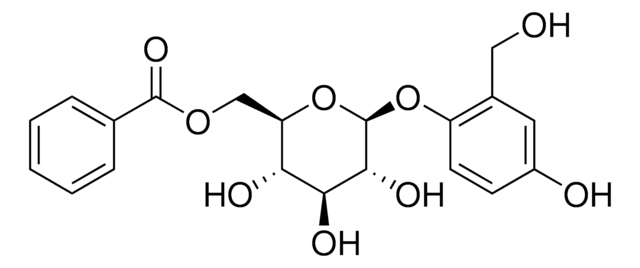
Stringa SMILE
O[C@H]1N2[C@@]([C@H](C)CC[C@H]2C3=COC=C3)([H])CC[C@@]14SC[C@]5(C4)CC[C@]([C@H](C)CC[C@H]6C7=COC=C7)([H])N6[C@@H]5O
InChI
1S/C30H42N2O4S/c1-19-3-5-25(21-9-13-35-15-21)31-23(19)7-11-29(27(31)33)17-30(37-18-29)12-8-24-20(2)4-6-26(32(24)28(30)34)22-10-14-36-16-22/h9-10,13-16,19-20,23-28,33-34H,3-8,11-12,17-18H2,1-2H3/t19-,20-,23+,24+,25+,26+,27-,28-,29-,30+/m1/s1
DYEOLAMWQVWASS-XKCSGWQSSA-N
Descrizione generale
Azioni biochim/fisiol
It was also found to act synergistically with cytotoxic drugs such as cisplatin and etoposide, enabling their cytotoxic effect at lower concentrations.[2]
6,6′-Dihydroxythiobinupharidine was found to have cytotoxic activity at a concentration of ~10 μM on human leukemia cells (U937), mouse melanoma cells (B16F10), and human fibroblasts (HT1080).[3]
In addition, Nuphar lutea extract was effective against both Leishmania promastigote and amastigote forms (IC50 = 2 ± 0.12 μg/mL; ID50 = 0.65 ± 0.023 μg/mL; LD50 = 2.1 ± 0.096 μg/mL, STI = 3.23). A synergistic antileishmanial activity was demonstrated with the antileishmanial drug, paromomycin.[4]
Recently 6,6′-dihydroxythiobinupharidine was found to be active against MRSA and VRE strains with an MIC of 1-4 μg/mL. Inhibition of DNA topoisomerase IV but not DNA gyrase in S. aureus was suggested as the mechanism of action.[5] 6,6′-Dihydroxythiobinupharidine was also shown to promote neutrophil effector bactericidal functions.[6]
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.