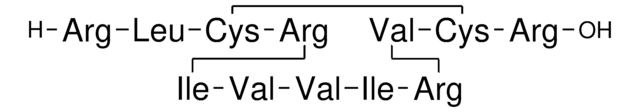C5241
Cinnamycin
from Streptomyces cinnamoneus, ≥95% (HPLC)
Sinonimo/i:
Lanthiopeptin, NSC-71936, Ro 09-0198
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Origine biologica
Streptomyces cinnamoneus
Livello qualitativo
Saggio
≥95% (HPLC)
Stato
solid
Solubilità
DMSO: 10 mg/mL
acetonitrile: water (1:1): 5 mg/mL (requires heating)
Spettro attività antibiotica
fungi
Modalità d’azione
cell membrane | interferes
Temperatura di conservazione
2-8°C
Stringa SMILE
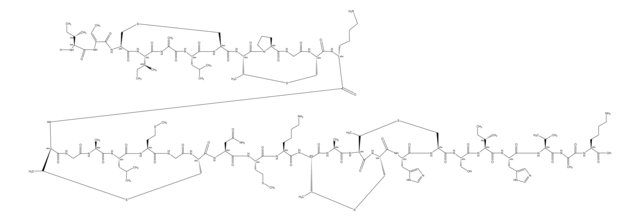
S1[C@@H](C2NC(=O)[C@@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H]3NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]5NC(=O)[C@@H](NC(=O)[C@H]7N(CCC7)C(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C1)N)CCCN\C(=N/[H])\N)CCC(=O)N)CS
InChI
1S/C89H125N25O25S3/c1-43(2)66-84(133)109-59-41-140-40-58-79(128)108-60-42-142-45(4)68(86(135)105-55(75(124)110-66)34-48-22-12-7-13-23-48)111-76(125)54(33-47-20-10-6-11-21-47)104-82(131)61-26-17-31-114(61)65(118)38-98-72(121)53(32-46-18-8-5-9-19-46)103-78(127)57(106-80(60)129)36-95-29-15-14-24-52(87(136)137)102-85(134)67(112-77(126)56(35-63(92)116)99-64(117)37-97-83(132)69(113-81(59)130)70(119)88(138)139)44(3)141-39-49(90)71(120)100-50(25-16-30-96-89(93)94)73(122)101-51(74(123)107-58)27-28-62(91)115/h5-13,18-23,43-45,49-61,66-70,95,119H,14-17,24-42,90H2,1-4H3,(H2,91,115)(H2,92,116)(H,97,132)(H,98,121)(H,99,117)(H,100,120)(H,101,122)(H,102,134)(H,103,127)(H,104,131)(H,105,135)(H,106,129)(H,107,123)(H,108,128)(H,109,133)(H,110,124)(H,111,125)(H,112,126)(H,113,130)(H,136,137)(H,138,139)(H4,93,94,96)/t44-,45?,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,66+,67?,68+,69+,70-/m1/s1
QJDWKBINWOWJNZ-IDGBIKHQSA-N
Categorie correlate
Amino Acid Sequence
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Cinnamycin, like other lantibiotics, was also reported to inhibit phospholipase A2 (PLA2). It was suggested as an alternative treatment for atherosclerosis through its ability to inhibit PLA2 by binding to its substrate PE. Moreover, Cinnamycin was found to inhibit Herpes simplex virus (HSV-1) activity.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Ribosomally synthesized antimicrobial peptides are a promising focus in antibiotic research amidst bacterial resistance and emerging infectious diseases.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.