80614
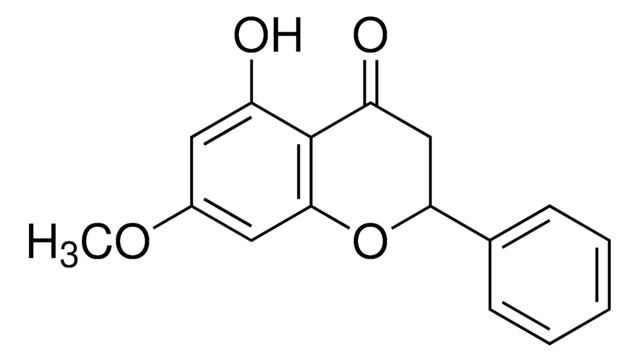
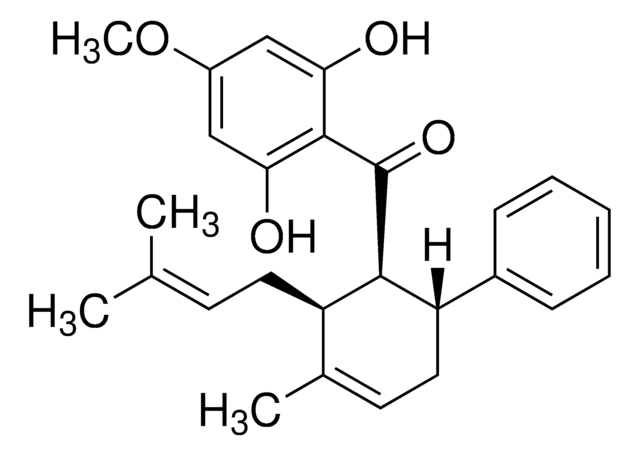
Pinostrobin
≥99.0% (TLC)
Sinonimo/i:
(S)-2,3-Dihydro-5-hydroxy-7-methoxy-2-phenyl-4H-1-benzopyran-4-one
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C16H14O4
Numero CAS:
Peso molecolare:
270.28
Beilstein:
270230
Numero CE:
Numero MDL:
Codice UNSPSC:
12352200
ID PubChem:
Prodotti consigliati
Saggio
≥99.0% (TLC)
Stringa SMILE
COc1cc(O)c2C(=O)C[C@H](Oc2c1)c3ccccc3
InChI
1S/C16H14O4/c1-19-11-7-12(17)16-13(18)9-14(20-15(16)8-11)10-5-3-2-4-6-10/h2-8,14,17H,9H2,1H3/t14-/m0/s1
ORJDDOBAOGKRJV-AWEZNQCLSA-N
Azioni biochim/fisiol
Elicits intense apoptotic response from cultured leukemia cells in vitro. Strongly inhibits the Ca2+ signals involved in the control of G2/M phase cell cycle progression in Saccharomyces cerevisiae. Shows potent antiviral effect against herpes simplex virus-1.
Confezionamento
Bottomless glass bottle. Contents are inside inserted fused cone.
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Nwet Nwet Win et al.
Journal of natural products, 70(10), 1582-1587 (2007-09-28)
The chloroform extract of rhizomes of Boesenbergia pandurata demonstrated marked preferential cytotoxicity against human pancreatic PANC-1 cancer cells in nutrient-deprived medium. Bioactivity-directed investigation of this extract yielded four new secondary metabolites, geranyl-2,4-dihydroxy-6-phenethylbenzoate ( 1), 2',4'-dihydroxy-3'-(1''-geranyl)-6'-methoxychalcone ( 2), (1' R,2' S,6'
J C Le Bail et al.
Cancer letters, 156(1), 37-44 (2000-06-07)
The interaction between the estrogen receptor and 5-hydroxy-7-methoxyflavanone (pinostrobin) was studied in the presence or absence of estradiol or dehydroepiandrosterone sulfate (DHEAS), respectively, using a stably transfected human breast cancer cell line (MVLN). We also evaluated its action on the
Tan Siew Kiat et al.
Bioorganic & medicinal chemistry letters, 16(12), 3337-3340 (2006-04-20)
Boesenbergia rotunda (L.) cyclohexenyl chalcone derivatives, 4-hydroxypanduratin A and panduratin A, showed good competitive inhibitory activities towards dengue 2 virus NS3 protease with the Ki values of 21 and 25 microM, respectively, whilst those of pinostrobin and cardamonin were observed
Hadi Poerwono et al.
Bioorganic & medicinal chemistry letters, 20(7), 2086-2089 (2010-03-12)
Pinostrobin (5-hydroxy-7-methoxyflavanone) obtained in relatively large amounts from fingerroot (Boesenbergia pandurata) was converted to its C-6 and C-8 prenylated derivatives. The Mitsunobu reaction, europium(III)-catalyzed Claisen-Cope rearrangement, and Claisen reaction coupled with cross-metathesis were used as the key steps. Using a
Chavi Yenjai et al.
Bioorganic & medicinal chemistry letters, 20(9), 2821-2823 (2010-04-07)
Flavones 1-4 isolated from Kaempferia parviflora were used for structural modification. Sixteen flavonoid derivatives, including four new derivatives, were synthesized and evaluated for cytotoxicity against KB and NCI-H187 cell lines. Flavanones 2a-4a demonstrated higher cytotoxic activity than the parent compounds.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.