20506
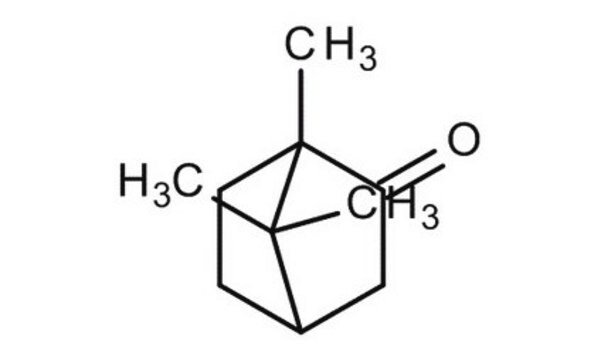
(±)-Camphor
meets analytical specification of Ph. Eur., BP, racemic, ≥95% (GC)
Sinonimo/i:
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one
About This Item
Prodotti consigliati
Densità del vapore
5.2 (vs air)
Livello qualitativo
Tensione di vapore
4 mmHg ( 70 °C)
Saggio
≥95% (GC)
Attività ottica
[α]20/D +0.15 to -0.15°, c = 10% in ethanol
Qualità
meets analytical specification of Ph. Eur., BP
racemic
Limite di esplosione
3.5 %
Impurezze
acidity or alcalinity, complies
related subst., complies (GC)
residual solvents, complies
water, complies
≤0.01% halogene compounds (as Cl)
≤0.05% non-volatile matter
P. eboll.
204 °C (lit.)
Punto di fusione
172-180 °C
175-177 °C (lit.)
Solubilità
carbon disulfide: freely soluble
hexane: freely soluble
liquid sulfur dioxide: soluble
phenol/1,2-dichlorobenzene: soluble
Compatibilità
complies for appearance of solution
passes test for identity
applicazioni
pharmaceutical (small molecule)
Stringa SMILE
[H][C@](CC1=O)(CC2)C(C)(C)[C@]12C
InChI
1S/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3/t7-,10+/m1/s1
DSSYKIVIOFKYAU-XCBNKYQSSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 2 Inhalation
Codice della classe di stoccaggio
4.1B - Flammable solid hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
147.9 °F - closed cup
Punto d’infiammabilità (°C)
64.4 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.