919888
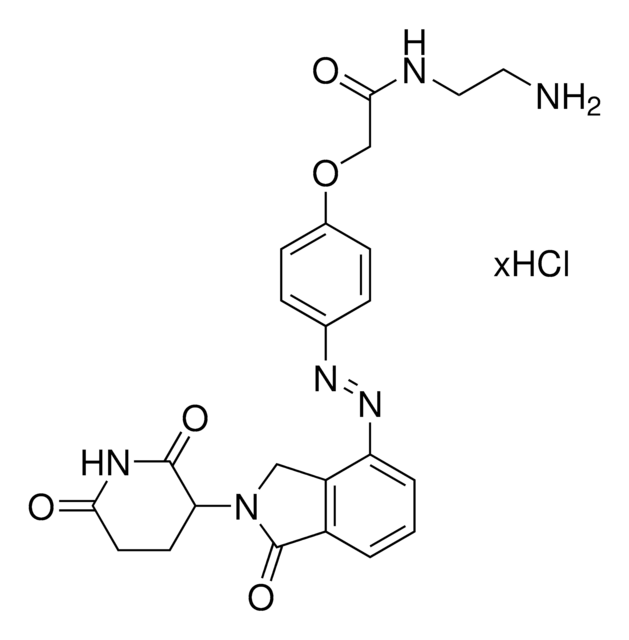
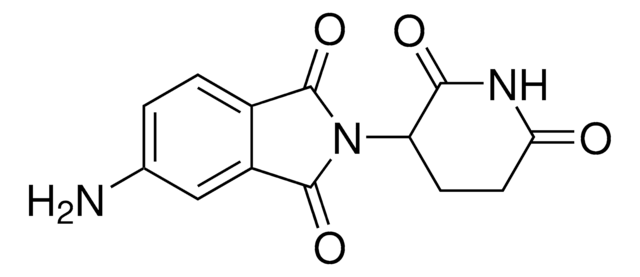
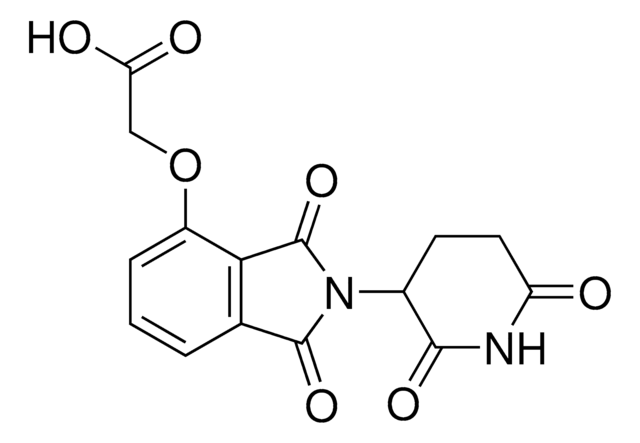
Thalidomide-Photoswitch3-NH2 hydrochloride
≥95%
Sinonimo/i:
(E)-N-(4-((4-Aminophenyl)diazenyl)phenyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamide hydrochloride, Photoswitchable protein degrader building block for PROTAC®
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
ligand
thalidomide
Livello qualitativo
Saggio
≥95%
Stato
solid
Impiego in reazioni chimiche
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
Gruppo funzionale
amine
Temperatura di conservazione
2-8°C
Stringa SMILE
O=C1N(C2C(NC(CC2)=O)=O)C(C3=C1C=CC=C3OCC(NC4=CC=C(/N=N/C5=CC=C(N)C=C5)C=C4)=O)=O.Cl
InChI
1S/C27H22N6O6.ClH/c28-15-4-6-17(7-5-15)31-32-18-10-8-16(9-11-18)29-23(35)14-39-21-3-1-2-19-24(21)27(38)33(26(19)37)20-12-13-22(34)30-25(20)36;/h1-11,20H,12-14,28H2,(H,29,35)(H,30,34,36);1H/b32-31+;
LEIFAZYMMHNBAT-MRRLHAJBSA-N
Categorie correlate
Applicazioni
Suggested wavelengths for photoswitching:
- Switch to cis isomer: 390 nm (380-400 nm)
- Switch to trans isomer (thermally more stable isomer): >450 nm
Browse our full offering of degrader building blocks that streamlines the synthesis of degrader libraries.
Learn more:
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Portal: Building PROTAC Degraders for Targeted Protein Degradation
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Altre note
Note legali
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.