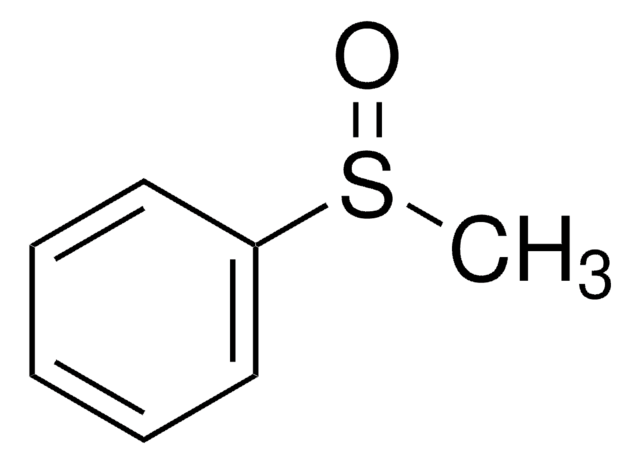
549304
4′-(Methylsulfonyl)acetophenone
97%
Sinonimo/i:
1-[4-(Methylsulfonyl)phenyl]ethan-1-one, 4-(Methylsulfonyl)acetophenone, NSC 403928
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3SO2C6H4COCH3
Numero CAS:
Peso molecolare:
198.24
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
Prodotti consigliati
Saggio
97%
Punto di fusione
126-129 °C (lit.)
Stringa SMILE
CC(=O)c1ccc(cc1)S(C)(=O)=O
InChI
1S/C9H10O3S/c1-7(10)8-3-5-9(6-4-8)13(2,11)12/h3-6H,1-2H3
KAVZYDHKJNABPC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
4′-(Methylsulfonyl)acetophenone may be used in the synthesis of:
- 3-(4-Methylsulfonylphenyl)-4-phenyl-2(5H)-furanone with potent apoptosis-inducing ability.
- Bromo-4-methylsulfonylacetophenone, an intermediate for preparing DL-threo-2-dichloroacetamido-1-(4-methylsulfonylphenyl)-1,3-propanediol.
- 1-N-Substituted-3,5-diphenyl-2-pyrazoline derivatives, which show promising anti-inflammatory activity.
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
New Antibacterial Agents. II. An Alternate Synthesis of DL-threo-2-Dichloro-acetamido-1-(4-methylsulfonylphenyl)-1, 3-propanediol1.
Suter CM, et al.
Journal of the American Chemical Society, 75(17), 4330-4333 (1953)
Jiuxiang Zhu et al.
Journal of the National Cancer Institute, 94(23), 1745-1757 (2002-12-05)
The cyclooxygenase-2 (COX-2) inhibitor celecoxib is thought to act as a chemopreventive agent by sensitizing cancer cells to apoptotic signals. Other COX-2 inhibitors, such as rofecoxib, are two orders of magnitude less potent than celecoxib at inducing apoptosis. The molecular
Rossella Fioravanti et al.
European journal of medicinal chemistry, 45(12), 6135-6138 (2010-10-27)
Eighteen new 1-N-substituted-3,5-diphenyl-2-pyrazoline derivatives have been synthesized and cyclooxygenase (COX-1 and COX-2) inhibitory activities have been evaluated. The results of these biological assays showed that all of new derivatives are not endowed with improved anti-inflammatory activity against COX-1, but some
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)