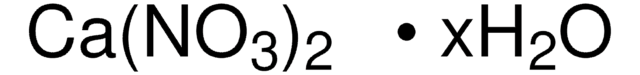288640
Manganese(II) nitrate hydrate
98%
Sinonimo/i:
Manganese(2+) dinitrate hydrate, Manganous dinitrate hydrate
About This Item
Prodotti consigliati
Saggio
98%
Forma fisica
solid
Composizione
Degree of hydration, 4-6
Temperatura di conservazione
2-8°C
Stringa SMILE
O.[Mn++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Mn.2NO3.H2O/c;2*2-1(3)4;/h;;;1H2/q+2;2*-1;
HBTFASPVVFSRRI-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- A precursor for the synthesis of manganese dioxide (MnO2), which is a common cathode material in primary alkaline batteries.
- A precursor in the synthesis of manganese dioxide (MnO2) for use in flexible micro supercapacitors. MnO2 exhibits high specific capacitance and excellent cycling stability, making it suitable for rapid energy storage applications.
- A manganese source in the colloidal solution combustion synthesis process to produce 3D δ-MnO2 nanostructures with ultra-large mesopores used in the fabrication of high-performance lithium-ion battery anodes.
- A precursor in the microwave-aided fabrication of calcium-substituted dysprosium perovskite manganite oxide nanocomposites (DyMnO3) for supercapacitor applications.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Ox. Sol. 3 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
5.1B - Oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.