254916
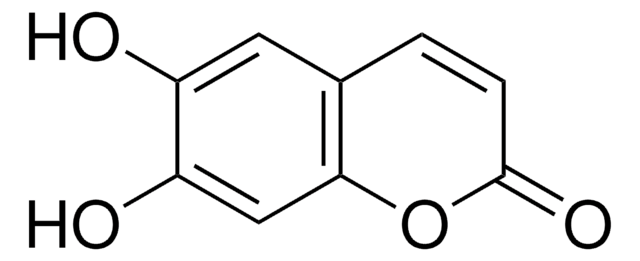
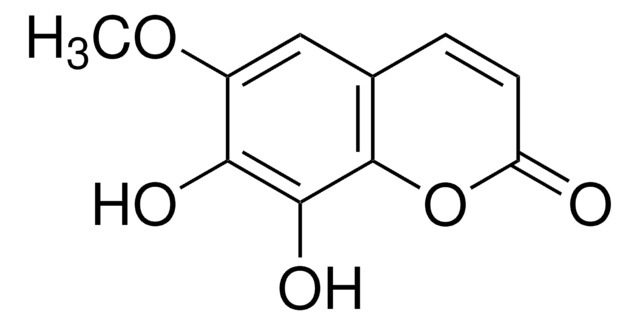
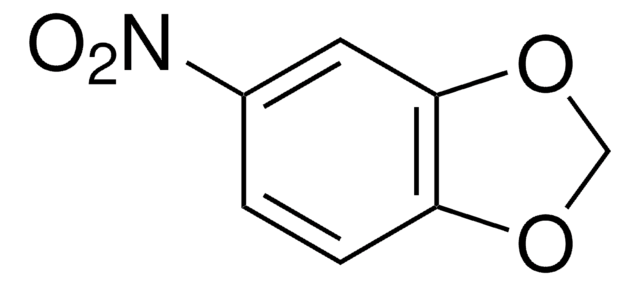
7,8-Dihydroxy-6-methoxycoumarin
98%
Sinonimo/i:
Fraxetin
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H8O5
Numero CAS:
Peso molecolare:
208.17
Numero CE:
Numero MDL:
Codice UNSPSC:
12162002
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
powder
Punto di fusione
230-231 °C (lit.)
Stringa SMILE
COc1cc2C=CC(=O)Oc2c(O)c1O
InChI
1S/C10H8O5/c1-14-6-4-5-2-3-7(11)15-10(5)9(13)8(6)12/h2-4,12-13H,1H3
HAVWRBANWNTOJX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
7,8-Dihydroxy-6-methoxycoumarin is a versatile compound known for its unique fluorescence properties and its role as a photoinitiator in polymerization processes. It exhibits high reactivity under UV light, making it an ideal candidate for applications in the polymerization industry, particularly in the formulation of coatings, adhesives, and inks. It is also used in biomedical applications, such as drug delivery due to its biocompatibility.
Applicazioni
7,8-Dihydroxy-6-methoxycoumarincan be used as a UV absorber in polymer formulations. Its ability to absorbultraviolet light helps in protecting polymers from degradation caused by UVradiation, thereby improving their longevity and stability.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
María Isabel Sánchez-Reus et al.
Neuroscience research, 53(1), 48-56 (2005-07-06)
Fraxetin belongs to an extensive group of natural phenolic anti-oxidants. In the present study, using a human neuroblastoma SH-SY5Y cells, we have investigated the protective effects of this compound on modifications in endogenous reduced glutathione (GSH), intracellular oxygen species (ROS)
Francesco Epifano et al.
Mini reviews in medicinal chemistry, 9(11), 1262-1271 (2009-11-26)
Neurodegenerative disorders are a heterogeneous group of diseases and are among the most invaliding syndromes for humans. The aim of this manuscript is to review what has been reported so far in the literature about coumarins as a novel class
Po-Lin Kuo et al.
Biological & pharmaceutical bulletin, 29(1), 119-124 (2006-01-06)
Fraxetin (7,8-dihydroxy-6-methoxy coumarin), a coumarin derivative, was investigated for its effects on differentiation of osteoblasts. By means of alkaline phosphatase (ALP) activity and osteocalcin ELISA assay, we have shown that fraxetin exhibits a significant induction of differentiation in two human
María Francisca Molina-Jiménez et al.
Brain research, 1009(1-2), 9-16 (2004-05-04)
Rotenone-induced apoptosis is considered to contribute to the etiology of Parkinson's disease (PD). We try to prevent the apoptosis induced by rotenone toxicity with 50 microM myricetin, 100 microM fraxetin and 100 microM N-acetylcysteine (NAC) that protect against reactive oxygen
Po-Lin Kuo et al.
International immunopharmacology, 6(7), 1167-1175 (2006-05-23)
The survival of osteoblast cells is one of the determinants of the development of osteoporosis in patients with inflamed synovium, such as in rheumatoid arthritis (RA). By means of alkaline phosphatase (ALP) activity and osteocalcin ELISA assay, we have shown
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.