218820
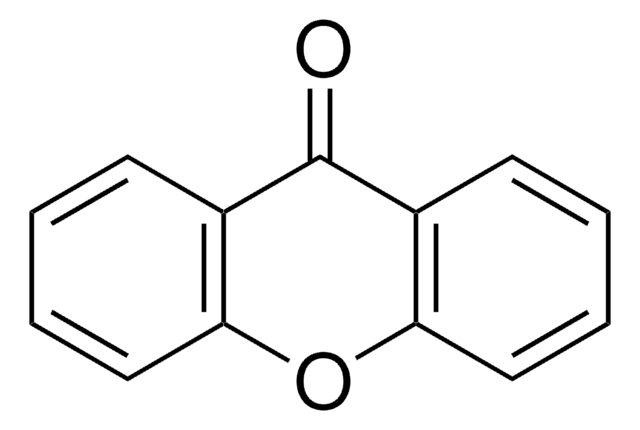
Phenoxathiin
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C12H8OS
Numero CAS:
Peso molecolare:
200.26
Beilstein:
143232
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
Prodotti consigliati
Saggio
97%
P. eboll.
150-152 °C/5 mmHg (lit.)
Punto di fusione
52-56 °C (lit.)
Stringa SMILE
O1c2ccccc2Sc3ccccc13
InChI
1S/C12H8OS/c1-3-7-11-9(5-1)13-10-6-2-4-8-12(10)14-11/h1-8H
GJSGGHOYGKMUPT-UHFFFAOYSA-N
Informazioni sul gene
rat ... Maoa(29253) , Maob(25750)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Aurica Varlan et al.
Molecules (Basel, Switzerland), 15(6), 3905-3919 (2010-07-27)
The interactions of 3-carboxyphenoxathiin with Bovine Serum Albumin (BSA) and Human Serum Albumin (HSA) have been studied by fluorescence and circular dichroism spectroscopy. The binding of 3-carboxyphenoxathiin quenches the BSA and HSA fluorescence, revealing a 1:1 interaction with a binding
A selective, reversible, competitive inhibitor of monoamine oxidase A containing no nitrogen, with negligible potentiation of tyramine-induced blood pressure rise.
M Harfenist et al.
Journal of medicinal chemistry, 34(9), 2931-2933 (1991-09-01)
L J Fitzgerald et al.
Acta crystallographica. Section C, Crystal structure communications, 47 ( Pt 2), 381-385 (1991-02-15)
Mr = 200.25, orthorhombic, P2(1)2(1)2(1), a = 7.758(2), b = 20.506(3), c = 5.896(2) A, V = 938.0(4) A3, Z = 4, Dx = 1.42 g cm-3, lambda(Mo K alpha) = 0.71069 A, mu = 2.88 cm-1, F(000) = 416
K Stolze et al.
Chemico-biological interactions, 77(3), 283-289 (1991-01-01)
Several derivatives of the phenothiazine cation radicals intercalated into DNA have been investigated using a new flow orientation technique. The anisotropic hyperfine coupling constants of both the parallel and the perpendicular orientation relative to the magnetic field were measured and
H Nojiri et al.
Journal of bacteriology, 181(10), 3105-3113 (1999-05-13)
Carbazole 1,9a-dioxygenase (CARDO) from Pseudomonas sp. strain CA10 is a multicomponent enzyme that catalyzes the angular dioxygenation of carbazole, dibenzofuran, and dibenzo-p-dioxin. It was revealed by gas chromatography-mass spectrometry and 1H and 13C nuclear magnetic resonance analyses that xanthene and
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.