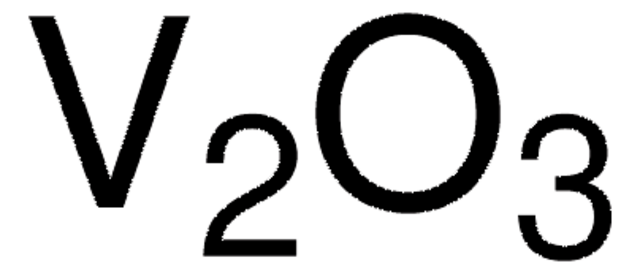215066
Gallium(III) oxide
≥99.99% trace metals basis
Sinonimo/i:
Gallium trioxide
About This Item
Prodotti consigliati
Saggio
≥99.99% trace metals basis
Forma fisica
(crystalline powder)
Impiego in reazioni chimiche
reagent type: catalyst
core: gallium
Densità
5.88 g/mL at 25 °C
Stringa SMILE
O=[Ga]O[Ga]=O
InChI
1S/2Ga.3O
QZQVBEXLDFYHSR-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
Due to its distinct optical and electrical properties like moderate conductivity and high laser damage threshold, Ga2O3 can be used in laser-driven electron accelerators, low-loss plasmonics, and Si-based dielectric laser accelerators.
It can also be used as an effective catalyst for the dehydrogenation of propane to propene.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.