130036
N-Methyl-4-piperidone
97%
Sinonimo/i:
1-Methyl-4-piperidinone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H11NO
Numero CAS:
Peso molecolare:
113.16
Beilstein:
106924
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Stato:
liquid
Saggio:
97%
Prodotti consigliati
Conformità normativa
suitable for FDA C-010.02
Livello qualitativo
Saggio
97%
Stato
liquid
Densità
0.92 g/mL at 25 °C (lit.)
Gruppo funzionale
ketone
Temperatura di conservazione
2-8°C
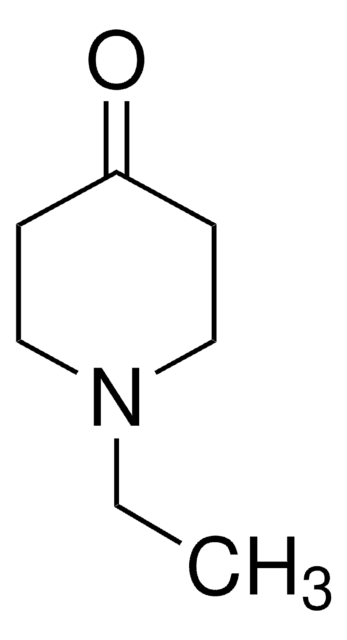
Stringa SMILE
CN1CCC(=O)CC1
InChI
1S/C6H11NO/c1-7-4-2-6(8)3-5-7/h2-5H2,1H3
HUUPVABNAQUEJW-UHFFFAOYSA-N
Applicazioni
N-Methyl-4-piperidone can be used as a reactant to prepare:
- Spiropiperidine rings by reacting with malononitrile and electrophiles or Michael acceptors.
- (3E,5E)-1-Methyl-3,5-bis(phenylmethylene)-4-piperidinone by reacting with benzaldehyde via Michael addition, followed by intramolecular O-cyclization/elimination sequential reactions.
- N,N′-Dimethylbispidinone by utilizing a double Mannich condensation method.
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Flam. Liq. 3
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
136.4 °F - closed cup
Punto d’infiammabilità (°C)
58 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Analogs of sparteine. I. Reexamination of the reaction of N-methyl-4-piperidone with formaldehyde and methylamine. Revised synthesis of N, N'-dimethylbispidinone
Smissman EE, et al.
The Journal of Organic Chemistry, 40, 251-252 (1975)
Novel route to spiropiperidines using N-methyl-4-piperidone, malononitrile and electrophiles
Lakshmi NV, et al.
Tetrahedron Letters, 53, 1282-1286 (2012)
Analogs of sparteine. I. A reexamination of the reaction of n-methyl-4-piperidone with formaldehyde and methylamine. A revised synthesis of n,n'-dimethylbispidinone.
E E Smissman et al.
The Journal of organic chemistry, 40(2), 251-252 (1975-01-24)
A facile tandem Michael addition/O-cyclization/elimination route to novel chromeno [3, 2-c] pyridines
Sumesh RV, et al.
Molecular Diversity, 19, 233-249 (2015)
Bin-Rong Yao et al.
European journal of medicinal chemistry, 167, 187-199 (2019-02-17)
To get new anti-hepatoma agents with anti-inflammatory activity and hypotoxicity, a series of dissymmetric pyridyl-substituted 3,5-bis(arylidene)-4-piperidones (BAPs, 25-82) were designed and synthesized. Many of them exhibited potential anti-hepatoma properties against human hepatocellular carcinoma cell lines (HepG2, QGY-7703, SMMC-7721) and hypotoxicity
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.