8.17076
TWEEN® 60 (Polysorbate)
EMPROVE® ESSENTIAL, Ph. Eur., JPE, NF
Fabricación farmacéutica
Sinónimos:
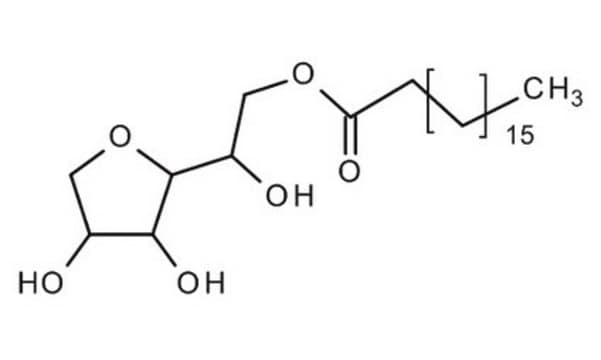
Polyoxyethylene sorbitan monostearate
About This Item
Productos recomendados
agency
JPE
NF
Ph. Eur.
Quality Level
vapor pressure
<10 hPa ( 20 °C)
product line
EMPROVE® ESSENTIAL
form
viscous liquid
technique(s)
API processing | nano-milling: suitable
pH
4,0-7.7 (25 °C, 50 g/L in H2O)
kinematic viscosity
170-320 cSt(40 °C)
transition temp
flash point >149 °C
density
1.1 g/cm3 at 20 °C
application(s)
liquid formulation
ophthalmics
pharma/biopharma processes
pharmaceutical
semi-solid formulation
solid formulation
solubility enhancement
storage temp.
15-25°C
InChI
1S/C26H50O10/c1-2-3-4-5-6-7-8-9-10-11-24(30)34-19-18-31-20-22(32-15-12-27)26-25(35-17-14-29)23(21-36-26)33-16-13-28/h22-23,25-29H,2-21H2,1H3
InChI key
HMFKFHLTUCJZJO-UHFFFAOYSA-N
General description
As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for biopharmaceutical and pharmaceutical formulation and production withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
Application
Legal Information
Application
related product
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Medicine for children poses unique formulation challenges compared to adults. Consider developmental physiology and age specifics when designing pharmaceuticals. Quality issues can severely impact patient safety. Therefore, excipient quality, supplier selection, and supply chain security are crucial, particularly for pediatric formulations.
Medicine for children poses unique formulation challenges compared to adults. Consider developmental physiology and age specifics when designing pharmaceuticals. Quality issues can severely impact patient safety. Therefore, excipient quality, supplier selection, and supply chain security are crucial, particularly for pediatric formulations.
Medicine for children poses unique formulation challenges compared to adults. Consider developmental physiology and age specifics when designing pharmaceuticals. Quality issues can severely impact patient safety. Therefore, excipient quality, supplier selection, and supply chain security are crucial, particularly for pediatric formulations.
Medicine for children poses unique formulation challenges compared to adults. Consider developmental physiology and age specifics when designing pharmaceuticals. Quality issues can severely impact patient safety. Therefore, excipient quality, supplier selection, and supply chain security are crucial, particularly for pediatric formulations.
Contenido relacionado
Developing formulations specifically for infants and children is increasingly important. To help you master these challenges effectively, we offer an extensive portfolio of well-established high-quality excipients and APIs for pediatric pharmaceutical formulations that are proven in practice.
Developing formulations specifically for infants and children is increasingly important. To help you master these challenges effectively, we offer an extensive portfolio of well-established high-quality excipients and APIs for pediatric pharmaceutical formulations that are proven in practice.
Developing formulations specifically for infants and children is increasingly important. To help you master these challenges effectively, we offer an extensive portfolio of well-established high-quality excipients and APIs for pediatric pharmaceutical formulations that are proven in practice.
Developing formulations specifically for infants and children is increasingly important. To help you master these challenges effectively, we offer an extensive portfolio of well-established high-quality excipients and APIs for pediatric pharmaceutical formulations that are proven in practice.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico