所有图片(2)
About This Item
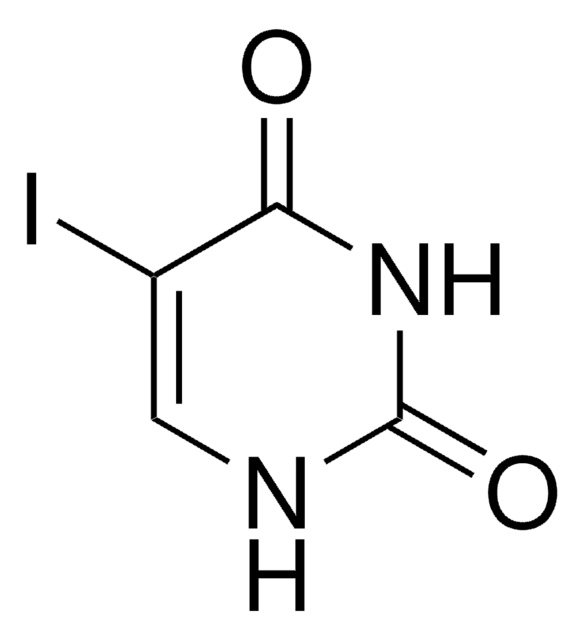
经验公式(希尔记法):
C4H4IN3O
CAS号:
分子量:
237.00
MDL编号:
UNSPSC代码:
41106305
PubChem化学物质编号:
NACRES:
NA.51
推荐产品
生物来源
synthetic (organic)
方案
≥99% (TLC)
表单
powder
溶解性
formic acid: 50 mg/mL
储存温度
−20°C
SMILES字符串
[H]N1C=C(I)C(N)=NC1=O
InChI
1S/C4H4IN3O/c5-2-1-7-4(9)8-3(2)6/h1H,(H3,6,7,8,9)
InChI key
UFVWJVAMULFOMC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
5-Iodocytosine is a modified pyrimidine used in the synthesis of molecules such as pyrrolocytosine and biologically active derivatives.
应用
5-Iodocytosine has been used as iodinated nucleotide with the single crystals of the 3D DNA designed motif for single anomalous dispersion (SAD) studies.
警示用语:
Warning
危险声明
危险分类
Acute Tox. 4 Oral
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
T S Heuer et al.
Biochemistry, 36(35), 10655-10665 (1997-09-02)
The virally encoded integrase protein carries out retroviral integration, and to do so, it must make specific interactions with both viral and target DNA sequences. The retroviral integrase has three domains: an amino-terminal region of about 50 amino acids that
Y Yoshimura et al.
Bioorganic & medicinal chemistry, 8(7), 1545-1558 (2000-09-08)
As part of our ongoing investigation of the synthesis of biologically interesting 2'-modified-4'-thionucleosides, we synthesized 2'-deoxy-2'-fluoro-4'-thioarabinofuranosylpyrimidine and -purine nucleosides, and evaluated their antiviral and antitumor activities. In the pyrimidine series, beta-anomers of 5-ethyluracil, 5-iodouracil, 5-chloroethyluracil, and 5-iodocytosine derivatives showed potent
R H E Hudson et al.
Nucleosides, nucleotides & nucleic acids, 24(5-7), 581-584 (2005-10-27)
We have employed a tandem Sonogashira/annulation reaction between 5-iodocytosine derivatives and terminal alkynes to yield the fluorescent bicyclic nucleobase pyrrolcytosine. Pyrrolocytosine bearing substituents only on the pyrrole ring are conveniently synthesized from 5-iodocytosine. Water soluble pyrrolocytosines are being investigated as
Chemistry for the synthesis of nucleobase-modified peptide nucleic acid
Hudson RHE, et al.
Pure and Applied Chemistry. Chimie Pure Et Appliquee, 76(7-8), 1591-1598 (2004)
Mouse RS21-C6 is a mammalian 2'-deoxycytidine 5'-triphosphate pyrophosphohydrolase that prefers 5-iodocytosine.
Nonaka M, Tsuchimoto D, et al.
Febs Letters, 276, 1654-1666 (2009)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门