推荐产品
生物源
Streptomyces sp.
品質等級
化驗
≥98% (HPLC)
形狀
powder
溶解度
methanol: 10 mg/mL, clear, colorless
儲存溫度
2-8°C
SMILES 字串
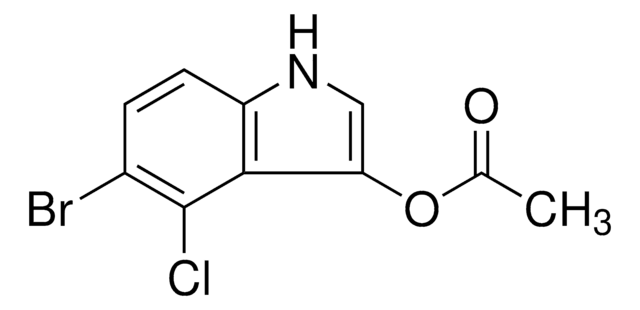
[H][C@@]1([C@H](C/C(C)=C/[C@H](C([C@H]([C@@H]([C@@H](CC)C)O)C)=O)C)C)OC([C@H]1C)=O
InChI
1S/C20H34O4/c1-8-12(3)17(21)15(6)18(22)13(4)9-11(2)10-14(5)19-16(7)20(23)24-19/h9,12-17,19,21H,8,10H2,1-7H3/b11-9+/t12-,13-,14+,15+,16+,17-,19+/m1/s1
InChI 密鑰
WOISDAHQBUYEAF-QIQXJRRPSA-N
一般說明
Esterase inhibitors produced by Streptomyces sp. MG7-G1 strain
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Amit K Mandal
Organic letters, 4(12), 2043-2045 (2002-06-07)
[structure: see text] The highly stereocontrolled hydroboration of an alkene, a subsequent Suzuki-Miyaura cross-coupling reaction, a silylcupration on a nonterminal acetylene, and an iododesilylation were the key steps in a convergent total synthesis of (-)-ebelactone A.
Low-molecular-weight immunomodifiers produced by micro-organisms.
H Umezawa
Biotechnology & genetic engineering reviews, 3, 255-273 (1985-01-01)
A Scaloni et al.
The Journal of biological chemistry, 269(21), 15076-15084 (1994-05-27)
The presence of a cysteine residue(s) near the active site of acylpeptide hydrolase was suggested by inactivation of the enzyme with sulfhydryl-modifying agents and by the substantial protection against inactivation afforded by the competitive inhibitor acetylmethionine. 5,5'-dithiobis-(2-nitrobenzoate) titrations of the
R Senthilkumar et al.
Experimental eye research, 72(3), 301-310 (2001-02-22)
Acylpeptide hydrolase removes the N -acetylated amino acids from the peptide substrates but not from intact proteins. Cleavage between amino acid residues 203--204 of the native acylpeptide hydrolase results in the formation of a 55 kDa truncated active enzyme in
Gianfranco De Pascale et al.
The Journal of antibiotics, 64(7), 483-487 (2011-04-28)
Homoserine transacetylase (HTA) catalyzes the transfer of an acetyl group from acetyl-CoA to the hydroxyl group of homoserine. This is the first committed step in the biosynthesis of methionine (Met) from aspartic acid in many fungi, Gram-positive and some Gram-negative
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门