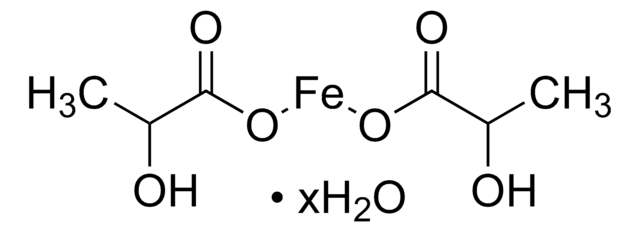
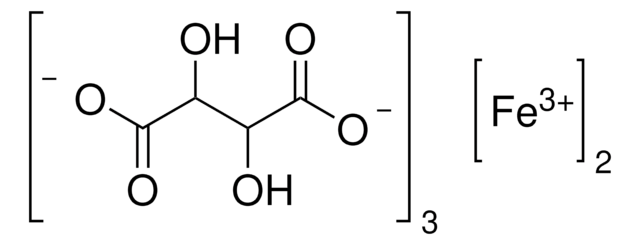
推荐产品
生物来源
synthetic
质量水平
无菌性
aseptically filled
方案
95-105 mg/mL (Iron content)
表单
solution
包含
0.5% phenol as preservative
组成
Iron, ~100 mg/mL
颜色
brown
有效pH范围
4 - 6.5
储存温度
room temp
SMILES字符串
[Fe].[S](=O)(=O)(O)O
InChI
1S/Fe.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)
InChI key
MVZXTUSAYBWAAM-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
警示用语:
Danger
危险声明
危险分类
Carc. 1B - Skin Sens. 1
储存分类代码
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Raymond J Bergeron et al.
Journal of medicinal chemistry, 51(13), 3913-3923 (2008-06-07)
A series of iron-clearing efficiencies (ICEs), ferrokinetics, and toxicity studies for ( S)-2-(2,4-dihydroxyphenyl)-4,5-dihydro-4-methyl-4-thiazolecarboxylic acid (deferitrin, 1), ( S)-4,5-dihydro-2-[2-hydroxy-4-(3,6,9-trioxadecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid ( 2), and (S)-4,5-dihydro-2-[2-hydroxy-3-(3,6,9-trioxadecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid ( 3) are reported. The ICEs in rodents are shown to be dose-dependent and saturable for
Natacha E Piloni et al.
Toxicology, 314(1), 174-182 (2013-10-15)
An in vivo model in rat was developed by intraperitoneally administration of Fe-dextran to study oxidative stress triggered by Fe-overload in rat brain. Total Fe levels, as well as the labile iron pool (LIP) concentration, in brain from rats subjected
Yonggang Gao et al.
Journal of ethnopharmacology, 145(1), 254-260 (2012-11-14)
Danshen (Salvia miltiorrhiza) has been widely prescribed in traditional folk medicine for treatment of hepatic and cardiovascular diseases in China and other Asian countries for several hundred years. Sixty male mice were randomly divided into five groups: control, iron overload
Zachariah DeFilipp et al.
Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery, 9(1), 129-132 (2012-08-08)
Iron deficiency is a major postoperative complication of Roux-en-Y gastric bypass surgery. Oral replacement can fail to correct the deficiency. Thus, recourse to parenteral iron administration might be necessary. Our objective was to evaluate the effectiveness and safety of a
Markus R Jahn et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 78(3), 480-491 (2011-03-29)
The treatment of iron deficiency anemia with polynuclear iron formulations is an established therapy in patients with chronic kidney disease but also in other disease areas like gastroenterology, cardiology, oncology, pre/post operatively and obstetrics' and gynecology. Parenteral iron formulations represent
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持