推荐产品
化驗
≥98% (HPLC)
形狀
powder
顏色
white
溶解度
DMSO: >10 mg/mL
起源
Sanofi Aventis
儲存溫度
2-8°C
SMILES 字串
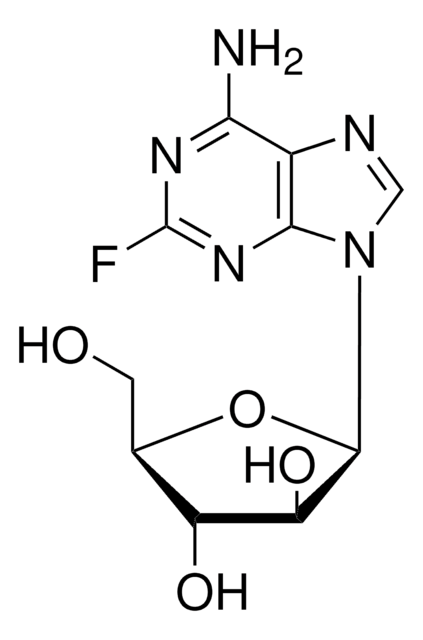
Nc1nc(Cl)nc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3F
InChI
1S/C10H11ClFN5O3/c11-10-15-7(13)5-8(16-10)17(2-14-5)9-4(12)6(19)3(1-18)20-9/h2-4,6,9,18-19H,1H2,(H2,13,15,16)/t3-,4+,6-,9-/m1/s1
InChI 密鑰
WDDPHFBMKLOVOX-AYQXTPAHSA-N
基因資訊
human ... POLA1(5422) , POLA2(23649) , POLD1(5424) , POLD2(5425) , POLD3(10714) , POLD4(57804) , POLE(5426) , POLE2(5427) , POLE3(54107) , PRIM1(5557) , PRIM2(5558) , RRM1(6240) , RRM2(6241) , RRM2B(50484)
應用
生化/生理作用
特點和優勢
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
商品
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Apoptosis regulation involves multiple pathways and molecules for cellular homeostasis.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门