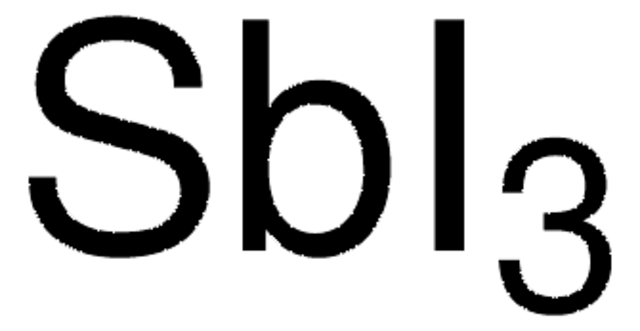推荐产品
方案
≥98%
储存温度
−20°C
SMILES字符串
[H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)CCCCCc5ccc6ccc7cccc8ccc5c6c78)[C@H](C)CCCC(C)C
InChI
1S/C49H64O2/c1-32(2)11-9-12-33(3)42-25-26-43-41-24-22-38-31-39(27-29-48(38,4)44(41)28-30-49(42,43)5)51-45(50)16-8-6-7-13-34-17-18-37-20-19-35-14-10-15-36-21-23-40(34)47(37)46(35)36/h10,14-15,17-23,32-33,39,41-44H,6-9,11-13,16,24-31H2,1-5H3/t33-,39+,41+,42-,43+,44+,48+,49-/m1/s1
InChI key
YRAVQIYIXJZCDI-JOMFGNKPSA-N
正在寻找类似产品? 访问 产品对比指南
储存分类代码
13 - Non Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
R Cazzola et al.
Journal of nutritional science and vitaminology, 45(1), 39-48 (1999-06-09)
The effect of alpha-tocopherol enrichment of low- and high-density lipoproteins on Cu(2+)-catalyzed lipid peroxidation in the hydrophobic core and in the hydrophilic envelope of lipoproteins was investigated by using two pyrene derivatives, namely, cholesteryl pyrenyl hexanoate (P6Chol) and pyrene dodecanoyl
A Joutti et al.
Chemistry and physics of lipids, 36(4), 335-341 (1985-03-01)
A fluorescent cholesterylester analogue, cholesteryl 6-pyrenylhexanoate (ChPH), was used as a substrate for pancreatic cholesterylester hydrolase (CEH, EC 3.1.1.13). The substrate consisted of ChPH in egg phosphatidylcholine stabilized microemulsion with the aqueous phase containing deoxycholate below its critical micellar concentration.
P Viani et al.
Biochimica et biophysica acta, 1315(2), 78-86 (1996-03-01)
Three different pyrene derivatives, pyrene decanoyl phosphatidylcholine (P10PC), pyrene dodecanoyl sulfatide (P12CS) and cholesteryl pyrenyl hexanoate (P6Chol), were used to follow lipid peroxidation in low and high density lipoproteins. Probe-labelled lipoproteins were subjected to Cu2+ catalyzed peroxidation. In all cases
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门