推荐产品
生物来源
rabbit
偶联物
unconjugated
抗体形式
IgG fraction of antiserum
抗体产品类型
primary antibodies
克隆
polyclonal
表单
PBS solution
分子量
antigen 42 kDa
种属反应性
human, mouse
技术
microarray: suitable
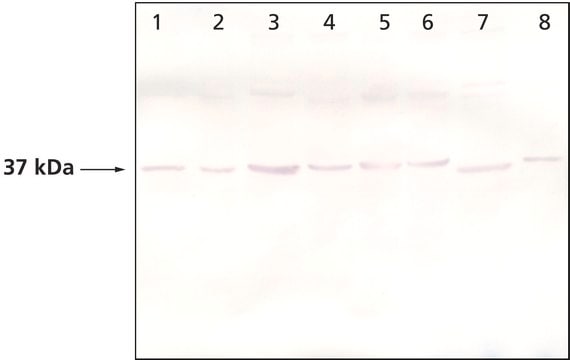
western blot: 1:3,000 using whole cell extract of the human endothelial ECV304 cell line.
UniProt登记号
运输
dry ice
储存温度
−20°C
靶向翻译后修饰
unmodified
基因信息
human ... PARVA(55742)
mouse ... Parva(57342)
一般描述
Actopaxin is a member of actin binding proteins that include beta-parvin/affixin and gamma-parvin. Actopaxin is known to associate with F-actin and paxillin LD motifs LD1 and LD4. This protein also directly binds to serine/threonine integrin-linked kinase (ILK). Studies have reported that actopaxin may be involved in integrin-dependent remodeling of the actin cytoskeleton during cell adhesion, motility and division.
Rabbit anti-actopaxin recognizes actopaxin (42 kDa). Staining of actopaxin in immunoblotting is specifically inhibited with actopaxin immunizing peptide (mouse, amino acids 35-53).
Rabbit anti-actopaxin recognizes actopaxin (42 kDa). Staining of actopaxin in immunoblotting is specifically inhibited with actopaxin immunizing peptide (mouse, amino acids 35-53).
免疫原
synthetic peptide corresponding to the N-terminal region of mouse actopaxin (amino acids 35-53). The sequence is identical in human actopaxin and has considerable homology (~70%) with β-parvin/affixin, but no homology with γ-parvin.
应用
Actopaxin was detected in HEK 293 cell lysates at 42 kDa by western blot using rabbit anti-actopaxin antibody at a 1:1000 dilution. In these cells, actopaxin associated with an ILK complex.
Applications in which this antibody has been used successfully, and the associated peer-reviewed papers, are given below.
Immunofluorescence (1 paper)
Immunofluorescence (1 paper)
Rabbit anti-actopaxin can also be used for protein microaarays.
外形
Solution in 0.01 M phosphate buffered saline, pH 7.4, containing 15 mM sodium azide
免责声明
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
未找到合适的产品?
试试我们的产品选型工具.
Sotiris N Nikolopoulos et al.
The Journal of biological chemistry, 277(2), 1568-1575 (2001-11-06)
Paxillin is a focal adhesion adapter protein involved in integrin signaling. We have recently reported that the paxillin LD1 motif acts as a binding interface for both the actin-binding protein actopaxin and the serine/threonine integrin-linked kinase (ILK). In this report
T M Olski et al.
Journal of cell science, 114(Pt 3), 525-538 (2001-02-15)
We have identified and cloned a novel 42-kDa protein termed alpha-parvin, which has a single alpha-actinin-like actin-binding domain. Unlike other members of the alpha-actinin superfamily, which are large multidomain proteins, alpha-parvin lacks a rod domain or any other C-terminal structural
Thitima Keskanokwong et al.
The Journal of biological chemistry, 282(32), 23205-23218 (2007-06-08)
Kidney anion exchanger 1 (kAE1) mediates chloride/bicarbonate exchange at the basolateral membrane of kidney alpha-intercalated cells, thereby facilitating bicarbonate reabsorption into the blood. Human kAE1 lacks the N-terminal 65 residues of the erythroid form (AE1, band 3), which are essential
S Yamaji et al.
The Journal of cell biology, 153(6), 1251-1264 (2001-06-13)
Focal adhesions (FAs) are essential structures for cell adhesion, migration, and morphogenesis. Integrin-linked kinase (ILK), which is capable of interacting with the cytoplasmic domain of beta1 integrin, seems to be a key component of FAs, but its exact role in
Iveta Dobreva et al.
Journal of proteome research, 7(4), 1740-1749 (2008-03-11)
Protein-protein interactions play an essential role in the regulation of vital biological functions. Through a network of interactions, integrin-linked kinase (ILK) functions downstream of integrin receptors to control cell spreading, migration, growth, survival, and cell cycle progression. Despite many reports
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门