推荐产品
等級
pharmaceutical primary standard
API 家族
medroxyprogesterone
製造商/商標名
EDQM
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
206-207 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
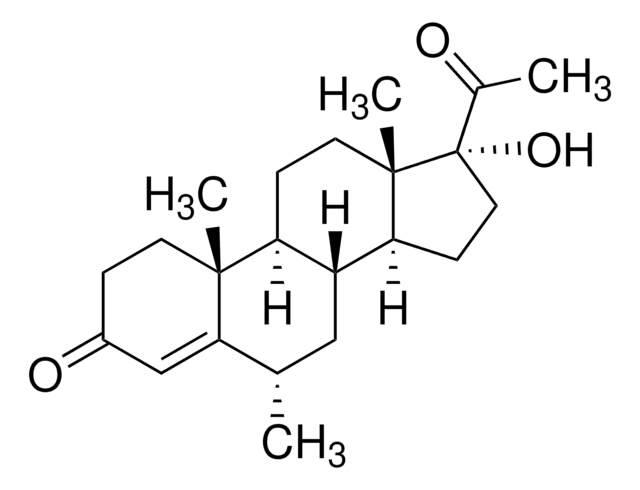
[H][C@@]12C[C@H](C)C3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@]4(C)[C@@]2([H])CC[C@]4(OC(C)=O)C(C)=O
InChI
1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1
InChI 密鑰
PSGAAPLEWMOORI-PEINSRQWSA-N
基因資訊
human ... PGR(5241)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Medroxyprogesterone acetate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 4 - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
其他客户在看
Jeong-Yeol Park et al.
Obstetrics and gynecology, 121(1), 136-142 (2012-12-25)
To analyze pregnancy outcomes in young women with stage IA, grade 1 endometrioid adenocarcinoma of the uterus after successful fertility-sparing management using progestin. We reviewed the medical records of 141 women with stage IA, grade 1 endometrioid adenocarcinoma of the
JoAnn E Manson et al.
JAMA, 310(13), 1353-1368 (2013-10-03)
Menopausal hormone therapy continues in clinical use but questions remain regarding its risks and benefits for chronic disease prevention. To report a comprehensive, integrated overview of findings from the 2 Women's Health Initiative (WHI) hormone therapy trials with extended postintervention
Lee L Lanza et al.
Obstetrics and gynecology, 121(3), 593-600 (2013-05-03)
Depot medroxyprogesterone acetate (DMPA) reversibly reduces bone mineral density. To estimate the extent to which DMPA might increase fracture risk, we undertook a retrospective cohort study of fractures in DMPA users and users of non-DMPA contraceptives, using the General Practice
Robert A Wild et al.
Menopause (New York, N.Y.), 20(3), 254-260 (2013-02-26)
Our objective was to determine whether metabolic syndrome (MetS) or its components modified the effect of hormone therapy (HT) on the risk of coronary heart disease (CHD) events in the Women's Health Initiative clinical trials. We performed a nested case-control
Richard P H Huijbregts et al.
Endocrinology, 154(3), 1282-1295 (2013-01-29)
Recent observational studies indicate an association between the use of hormonal contraceptives and acquisition and transmission of HIV-1. The biological and immunological mechanisms underlying the observed association are unknown. Depot medroxyprogesterone acetate (DMPA) is a progestin-only injectable contraceptive that is
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门