推荐产品
品質等級
化驗
≥98.0% (sum of enantiomers, GC)
光學活性
[α]20/D −67±2°, c = 2.3% in chloroform
mp
49-51 °C
SMILES 字串
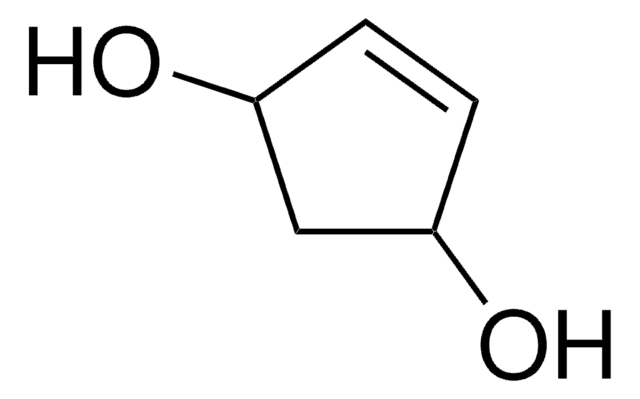
CC(=O)O[C@H]1C[C@@H](O)C=C1
InChI
1S/C7H10O3/c1-5(8)10-7-3-2-6(9)4-7/h2-3,6-7,9H,4H2,1H3/t6-,7+/m0/s1
InChI 密鑰
IJDYOKVVRXZCFD-NKWVEPMBSA-N
應用
(1R,4S)-cis-4-Acetoxy-2-cyclopenten-1-ol can be used as a reactant in the total synthesis of Bartlett′s brefeldin intermediate, spinosyn A analogs, (+)-7-deaza-5′-noraristeromycin, (±) strychnine and the Wieland-Gumlich aldehyde.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Asymmetric Total Syntheses of (-)-and (+)-Strychnine and the Wieland-Gumlich Aldehyde
Knight SD, et al.
Journal of the American Chemical Society, 117(21), 5776-5788 (1995)
A new approach to (+)-brefeldin A via a nickel-catalyzed coupling reaction of cyclopentenyl acetate and lithium 2-furylborate
Kobayashi Y, et al.
Tetrahedron Letters, 37(34), 6125-6128 (1996)
Synthesis of novel spinosyn A analogues by Pd-mediated transformations
Tietze LF, et al.
Chemistry?A European Journal , 13(30), 8543-8563 (2007)
(+)-7-Deaza-5 `-noraristeromycin as an anti-trypanosomal agent
Seley KL, et al.
Journal of Medicinal Chemistry, 40(4), 622-624 (1997)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门